Alternative nuclear transport for cellular protein quality control - PubMed (original) (raw)
Alternative nuclear transport for cellular protein quality control
April Rose et al. Trends Cell Biol. 2012 Oct.
Abstract
Herpesvirus capsids traverse the nuclear envelope (NE) by utilizing an unusual export pathway termed nuclear egress. In this process, the viral capsid is delivered into the perinuclear space (PNS), producing a vesicular intermediate after fission. After fusion with the outer nuclear membrane (ONM), the naked capsid is released into the cytosol. A recent study now suggests that this pathway might be an endogenous cellular pathway, co-opted by viruses, that serves to transport cellular cargo exceeding the size limit imposed by the nuclear pore complex (NPC). We propose that one function of this pathway is to transport nuclear protein aggregates to the cytosolic autophagy machinery. Our model has implications for our understanding of laminopathies and related diseases affecting proteins residing at the inner nuclear membrane (INM) and nuclear lamina.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
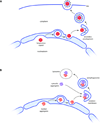
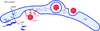
Figure 1
Egress of herpesvirus capsids from the nucleus: A paradigm for the delivery of nuclear protein aggregates to the cytosolic autophagy machinery? (a). Herpesvirus capsids are assembled in the nucleus and bud into the perinuclear space to form a membrane-enveloped intermediate, which then fuses with the outer nuclear membrane (ONM) to release the naked capsid into the cytosol, where the glycoprotein coat is acquired. (b). Using the same route, nuclear aggregates may be transported to the autophagic machinery that is confined to the cytosol (note that secondary envelopment, depicted in a, is topologically equivalent to isolation membrane formation at the onset of autophagy, depicted in b). The link to autophagy is at present hypothetical and awaits experimental validation (see main text and Box 1).
Figure 2
Manipulation of the nuclear envelope during viral egress. Dissolution of the nuclear lamina by local phosphorylation of nuclear lamins via kinases (i) enables the capsid to gain access to the inner nuclear membrane, and to dock onto the nuclear egress complex (ii). Budding (iii) and fission (iv) produce a vesicular intermediate, which fuses the outer nuclear membrane in a reaction that may involve gB (v). Lmn, Lamins; LBR, Lamin B receptor; PKC, protein kinase C; gB, Glycoprotein B. Cellular proteins are depicted in blue, viral proteins in red/orange. Note that the capsid is not drawn to scale: its diameter is approximately three times the width of the perinuclear space (PNS).
Similar articles
- Nuclear Egress of Herpesviruses: The Prototypic Vesicular Nucleocytoplasmic Transport.
Hellberg T, Paßvogel L, Schulz KS, Klupp BG, Mettenleiter TC. Hellberg T, et al. Adv Virus Res. 2016;94:81-140. doi: 10.1016/bs.aivir.2015.10.002. Epub 2016 Jan 29. Adv Virus Res. 2016. PMID: 26997591 Review. - Integrity of the Linker of Nucleoskeleton and Cytoskeleton Is Required for Efficient Herpesvirus Nuclear Egress.
Klupp BG, Hellberg T, Granzow H, Franzke K, Dominguez Gonzalez B, Goodchild RE, Mettenleiter TC. Klupp BG, et al. J Virol. 2017 Sep 12;91(19):e00330-17. doi: 10.1128/JVI.00330-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28724767 Free PMC article. - The Knowns and Unknowns of Herpesvirus Nuclear Egress.
Klupp BG, Mettenleiter TC. Klupp BG, et al. Annu Rev Virol. 2023 Sep 29;10(1):305-323. doi: 10.1146/annurev-virology-111821-105518. Epub 2023 Apr 11. Annu Rev Virol. 2023. PMID: 37040797 Review. - Homeostatic restitution of cell membranes. Nuclear membrane lipid biogenesis and transport of protein from cytosol to intranuclear spaces.
Slomiany A, Grabska M, Slomiany BL. Slomiany A, et al. Int J Biol Sci. 2006 Aug 30;2(4):216-26. doi: 10.7150/ijbs.2.216. Int J Biol Sci. 2006. PMID: 16967103 Free PMC article. - The way out: what we know and do not know about herpesvirus nuclear egress.
Mettenleiter TC, Müller F, Granzow H, Klupp BG. Mettenleiter TC, et al. Cell Microbiol. 2013 Feb;15(2):170-8. doi: 10.1111/cmi.12044. Epub 2012 Nov 7. Cell Microbiol. 2013. PMID: 23057731 Review.
Cited by
- CLCC1 promotes membrane fusion during herpesvirus nuclear egress.
Dai B, Polack L, Sperl A, Dame H, Huynh T, Deveney C, Lee C, Doench JG, Heldwein EE. Dai B, et al. bioRxiv [Preprint]. 2024 Sep 23:2024.09.23.614151. doi: 10.1101/2024.09.23.614151. bioRxiv. 2024. PMID: 39386602 Free PMC article. Preprint. - Methodologies to monitor protein turnover at the inner nuclear membrane.
Tsai PL, Zhao C, Schlieker C. Tsai PL, et al. Methods Enzymol. 2019;619:47-69. doi: 10.1016/bs.mie.2018.12.033. Epub 2019 Feb 8. Methods Enzymol. 2019. PMID: 30910029 Free PMC article. - Nuclear envelope budding is a response to cellular stress.
Panagaki D, Croft JT, Keuenhof K, Larsson Berglund L, Andersson S, Kohler V, Büttner S, Tamás MJ, Nyström T, Neutze R, Höög JL. Panagaki D, et al. Proc Natl Acad Sci U S A. 2021 Jul 27;118(30):e2020997118. doi: 10.1073/pnas.2020997118. Proc Natl Acad Sci U S A. 2021. PMID: 34290138 Free PMC article. - Cytoplasmic capes are nuclear envelope intrusions that are enriched in endosomal proteins and depend upon βH-spectrin and Annexin B9.
Wu J, Bakerink KJ, Evangelista ME, Thomas GH. Wu J, et al. PLoS One. 2014 Apr 4;9(4):e93680. doi: 10.1371/journal.pone.0093680. eCollection 2014. PLoS One. 2014. PMID: 24705398 Free PMC article. - How lamina-associated polypeptide 1 (LAP1) activates Torsin.
Sosa BA, Demircioglu FE, Chen JZ, Ingram J, Ploegh HL, Schwartz TU. Sosa BA, et al. Elife. 2014 Aug 22;3:e03239. doi: 10.7554/eLife.03239. Elife. 2014. PMID: 25149450 Free PMC article.
References
- Fields BN, et al. Fields' virology. 5th edn. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007.
- Johnson DC, Baines JD. Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol. 2011;9:382–394. - PubMed
- Nakatogawa H, et al. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. - PubMed
- Mettenleiter TC, et al. Herpesvirus assembly: a tale of two membranes. Curr Opin Microbiol. 2006;9:423–429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources