Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis - PubMed (original) (raw)
Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis
Mary B Goldring. Ther Adv Musculoskelet Dis. 2012 Aug.
Abstract
Chondrogenesis occurs as a result of mesenchymal cell condensation and chondroprogenitor cell differentiation. Following chondrogenesis, the chondrocytes remain as resting cells to form the articular cartilage or undergo proliferation, terminal differentiation to chondrocyte hypertrophy, and apoptosis in a process termed endochondral ossification, whereby the hypertrophic cartilage is replaced by bone. Human adult articular cartilage is a complex tissue of matrix proteins that varies from superficial to deep layers and from loaded to unloaded zones. A major challenge to efforts to repair cartilage by stem cell-based and other tissue-engineering strategies is the inability of the resident chondrocytes to lay down a new matrix with the same properties as it had when it was formed during development. Thus, understanding and comparing the mechanisms of cartilage remodeling during development, osteoarthritis (OA), and aging may lead to more effective strategies for preventing cartilage damage and promoting repair. The pivotal proteinase that marks OA progression is matrix metalloproteinase 13 (MMP-13), the major type II collagen-degrading collagenase, which is regulated by both stress and inflammatory signals. We and other investigators have found that there are common mediators of these processes in human OA cartilage. We also observe temporal and spatial expression of these mediators in early through late stages of OA in mouse models and are analyzing the consequences of knockout or transgenic overexpression of critical genes. Since the chondrocytes in adult human cartilage are normally quiescent and maintain the matrix in a low turnover state, understanding how they undergo phenotypic modulation and promote matrix destruction and abnormal repair in OA may to lead to identification of critical targets for therapy to block cartilage damage and promote effective cartilage repair.
Keywords: articular cartilage; chondrogenesis; inflammation; mouse models; osteoarthritis.
Figures
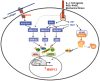
Figure 1.
Signaling pathways that converge on matrix metalloproteinase13 (MMP13) gene transcription in chondrocytes. Binding of the receptor tyrosine kinase, discoidin domain receptor (DDR) 2, to native type II collagen results in activation of RAS/RAF/MEK/extracellular-regulated kinase (ERK) signaling in a manner independent of integrin- or cytokine-induced signaling. Interleukin (IL)-1, toll-like receptor (TLR) ligand, reactive oxygen species (ROS), advanced glycation endproducts (AGEs) interact with the cell through distinct receptors that transduce phosphorylation events via cytoplasmic interactions initiating various protein kinase cascades. The major pathways involve activation of MAP triple kinases (MTK), MAP kinase kinases (MKKs) 3/6 and 4/7, and p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK), which lead to activation of activator protein 1 (AP-1) (cFos/cJun), Runx2, E Twenty Six (ETS) factors, HIF2α, and C/EBPβ, among other transcription factors; and inhibitor of κB (IκB) kinases (IKK) α and β, leading to activation of nuclear factor kappa B (NFκB) and its translocation to the nucleus. The responses of the target gene, MMP13, depend on the presence of DNA sequences within its promoter that bind to the various transcription factors.
Figure 2.
Strategies for studying mechanisms of osteoarthritis. The upper left panels show Safranin O/Fast green-stained human cartilage sections from a normal individual and a patient with osteoarthritis (OA) (arrows mark surface fibrillations and duplicated tidemark, derived from the tibial plateau [lower left panel] of a patient who underwent total knee replacement surgery (the medial side on the right is more affected than the lateral side). The upper right panels show Safranin O/Fast green-stained murine cartilage sections from knee joints left unoperated (control) or subjected to destabilization of the medial meniscus (DMM) surgery. Differentially regulated proteins and genes are identified in both the clinical material and the preclinical model and mechanisms are further studied in cell culture models of isolated chondrocytes in three-dimensional pellet or high-density monolayers.
Similar articles
- Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis.
Goldring MB, Otero M, Plumb DA, Dragomir C, Favero M, El Hachem K, Hashimoto K, Roach HI, Olivotto E, Borzì RM, Marcu KB. Goldring MB, et al. Eur Cell Mater. 2011 Feb 24;21:202-20. doi: 10.22203/ecm.v021a16. Eur Cell Mater. 2011. PMID: 21351054 Free PMC article. - Similar properties of chondrocytes from osteoarthritis joints and mesenchymal stem cells from healthy donors for tissue engineering of articular cartilage.
Fernandes AM, Herlofsen SR, Karlsen TA, Küchler AM, Fløisand Y, Brinchmann JE. Fernandes AM, et al. PLoS One. 2013 May 9;8(5):e62994. doi: 10.1371/journal.pone.0062994. Print 2013. PLoS One. 2013. PMID: 23671648 Free PMC article. - Cartilage degradation in osteoarthritis: A process of osteochondral remodeling resembles the endochondral ossification in growth plate?
Xiao ZF, Su GY, Hou Y, Chen SD, Lin DK. Xiao ZF, et al. Med Hypotheses. 2018 Dec;121:183-187. doi: 10.1016/j.mehy.2018.08.023. Epub 2018 Aug 27. Med Hypotheses. 2018. PMID: 30396477 - [RESEARCH PROGRESS OF PATHOLOGY OF ENDOCHONDRAL OSSIFICATION IN OSTEOARTHRITIS].
Xiao Z, Lin D. Xiao Z, et al. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016 Dec 8;30(12):1556-1561. doi: 10.7507/1002-1892.20160320. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016. PMID: 29786351 Review. Chinese. - Collagen type X expression and chondrocyte hypertrophic differentiation during OA and OS development.
Han T, Zhu T, Lu Y, Wang Q, Bian H, Chen J, Qiao L, He TC, Zheng Q. Han T, et al. Am J Cancer Res. 2024 Apr 15;14(4):1784-1801. doi: 10.62347/JWGW7377. eCollection 2024. Am J Cancer Res. 2024. PMID: 38726262 Free PMC article. Review.
Cited by
- Gene Expression and Chondrogenic Potential of Cartilage Cells: Osteoarthritis Grade Differences.
Mazor M, Lespessailles E, Best TM, Ali M, Toumi H. Mazor M, et al. Int J Mol Sci. 2022 Sep 13;23(18):10610. doi: 10.3390/ijms231810610. Int J Mol Sci. 2022. PMID: 36142513 Free PMC article. - Sclerostin and skeletal health.
Sharifi M, Ereifej L, Lewiecki EM. Sharifi M, et al. Rev Endocr Metab Disord. 2015 Jun;16(2):149-56. doi: 10.1007/s11154-015-9311-6. Rev Endocr Metab Disord. 2015. PMID: 25669441 Review. - Mesenchymal Stem Cell-Derived Exosomes as a Treatment Option for Osteoarthritis.
Vadhan A, Gupta T, Hsu WL. Vadhan A, et al. Int J Mol Sci. 2024 Aug 23;25(17):9149. doi: 10.3390/ijms25179149. Int J Mol Sci. 2024. PMID: 39273098 Free PMC article. Review. - Study on the Role of MicroRNA-214 in the Rehabilitation of Cartilage in Mice with Exercise-Induced Traumatic Osteoarthritis.
Cao H, Zhou X, Li H, Wang M, Wu W, Zou J. Cao H, et al. Curr Issues Mol Biol. 2022 Sep 7;44(9):4100-4117. doi: 10.3390/cimb44090281. Curr Issues Mol Biol. 2022. PMID: 36135193 Free PMC article. - Bibliometric analysis of the inflammatory mechanisms in knee osteoarthritis in recent 30 years.
Chen Y, Yu B, He F, Sun W, Song Y. Chen Y, et al. Am J Clin Exp Immunol. 2023 Dec 15;12(6):127-139. eCollection 2023. Am J Clin Exp Immunol. 2023. PMID: 38187364 Free PMC article.
References
- Aigner T., Fundel K., Saas J., Gebhard P.M., Haag J., Weiss T., et al. (2006) Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum 54: 3533–3544 - PubMed
- Alsalameh S., Amin R., Gemba T., Lotz M. (2004) Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum 50: 1522–1532 - PubMed
- Anderson H.C., Mulhall D., Garimella R. (2010) Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab Invest 90: 1549–1557 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources