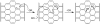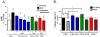Antioxidant carbon particles improve cerebrovascular dysfunction following traumatic brain injury - PubMed (original) (raw)
. 2012 Sep 25;6(9):8007-14.
doi: 10.1021/nn302615f. Epub 2012 Aug 15.
Affiliations
- PMID: 22866916
- PMCID: PMC3458163
- DOI: 10.1021/nn302615f
Antioxidant carbon particles improve cerebrovascular dysfunction following traumatic brain injury
Brittany R Bitner et al. ACS Nano. 2012.
Abstract
Injury to the neurovasculature is a feature of brain injury and must be addressed to maximize opportunity for improvement. Cerebrovascular dysfunction, manifested by reduction in cerebral blood flow (CBF), is a key factor that worsens outcome after traumatic brain injury (TBI), most notably under conditions of hypotension. We report here that a new class of antioxidants, poly(ethylene glycol)-functionalized hydrophilic carbon clusters (PEG-HCCs), which are nontoxic carbon particles, rapidly restore CBF in a mild TBI/hypotension/resuscitation rat model when administered during resuscitation--a clinically relevant time point. Along with restoration of CBF, there is a concomitant normalization of superoxide and nitric oxide levels. Given the role of poor CBF in determining outcome, this finding is of major importance for improving patient health under clinically relevant conditions during resuscitative care, and it has direct implications for the current TBI/hypotension war-fighter victims in the Afghanistan and Middle East theaters. The results also have relevancy in other related acute circumstances such as stroke and organ transplantation.
Figures
Figure 1
The suggested radical annihilation mechanism at a graphitic domain of the carbon particle. Two additions of ROG result in the loss of two C–C pi-bonds and the formation of two new C–O sigma bonds and one new C–C pi bond, without any radical species remaining. Only one regioisomer is shown, though many others can form by resonance of the conjugated radical.
Figure 2
PEG-HCCs protect brain endothelial cells from oxidative stress when administered after an insult. (A) Intracellular ROS levels for b.End3 cells as determined by DHE staining and flow cytometry. The mean fluorescent intensity (MFI) of 10,000 cells/group was normalized to the group not treated with antimycin A (AntA in graphs) or DHE. Phosphate buffered saline (PBS, black bar) or antioxidant treatments (blue, green, and red solid bars) were given after antimycin A. Some antioxidants were administered prior to antimycin A (striped bars). Additional controls are shown in Figure S2. * p-value < 0.05; ** p-value < 0.01; *** p-value < 0.001 compared to bar with arrow. Results are a mean of five separate experiments. (B) Cell survival relative to control for b.End3 cells given different treatments. The cells were either cultured in the presence of PEG-HCCs, PEG-SOD or PBN alone (solid bars) or the cells were first treated with a dose of antimycin A titrated to kill 30% of the cells followed by treatment with PEG-HCCs, PEG-SOD or PBN (striped bars). Results are a mean of seven separate experiments. Error bars are s.e.m. ANOVA with Bonferroni post test was used to calculate statistics. * p-value <0.05.
Figure 3
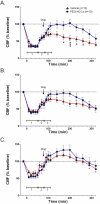
PEG-HCC treatment rapidly improves CBF in the injured cortex in rats with TBI plus hypotension after a single dose and two sequential doses. Laser Doppler was used to measure CBF given as a percent of the baseline (pre-injury) CBF. Relative CBF below 100 (dashed line) indicate low CBF. Relative CBF after a single drug dose (arrow) administered during the “hospital phase” in the (A) injured cortex (B) peri-lesional cortex, and (C) contralateral cortex; the effect of sequential dosing in a second set of rats treated with drug during the “hospital phase” and again 2 h later (arrows) are shown in (D) injured cortex (E) peri-lesional cortex, and (F) contralateral cortex. Drugs (PEG-HCC or PBS vehicle) were given where indicated. Time 0 min indicates when the TBI was performed. Phase 1 = TBI+hypotension; Phase 2 = saline during “ambulatory phase”; Phase 3 = vehicle or PEG-HCC treatment and blood reinfusion during “hospital phase”. Error bars are S.E.M. Repeated measures ANOVA with Bonferroni post test was used to calculate statistics. * p-value <0.05 and *** p-value <0.01 for vehicle (red) compared to PEG-HCCs (blue). Note that 100% of CBF is considered “normal”. Approximately 50% and 150% are considered low and high cerebral blood flow, respectively.
Figure 3
PEG-HCC treatment rapidly improves CBF in the injured cortex in rats with TBI plus hypotension after a single dose and two sequential doses. Laser Doppler was used to measure CBF given as a percent of the baseline (pre-injury) CBF. Relative CBF below 100 (dashed line) indicate low CBF. Relative CBF after a single drug dose (arrow) administered during the “hospital phase” in the (A) injured cortex (B) peri-lesional cortex, and (C) contralateral cortex; the effect of sequential dosing in a second set of rats treated with drug during the “hospital phase” and again 2 h later (arrows) are shown in (D) injured cortex (E) peri-lesional cortex, and (F) contralateral cortex. Drugs (PEG-HCC or PBS vehicle) were given where indicated. Time 0 min indicates when the TBI was performed. Phase 1 = TBI+hypotension; Phase 2 = saline during “ambulatory phase”; Phase 3 = vehicle or PEG-HCC treatment and blood reinfusion during “hospital phase”. Error bars are S.E.M. Repeated measures ANOVA with Bonferroni post test was used to calculate statistics. * p-value <0.05 and *** p-value <0.01 for vehicle (red) compared to PEG-HCCs (blue). Note that 100% of CBF is considered “normal”. Approximately 50% and 150% are considered low and high cerebral blood flow, respectively.
Figure 4
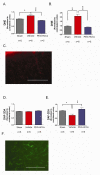
Fluorescence after systemic injection of radical-sensitive dyes measured in the brain and vasculature following TBI. DHE levels (proportional to SO and oxidative radicals) in the (A) brain parenchyma (cortex) and (B) blood vessels of sham (no TBI) surgery rats or rats with TBI + hypotension with vehicle or PEG-HCC treatment. The ipsilateral cortex (brain hemisphere with TBI) was normalized to the contralateral cortex. PEG-HCC treatment was able to reduce DHE staining (blue column) compared to vehicle treatment (red column), with a larger magnitude of effect seen in the blood vessels than the brain parenchyma, suggesting that the cerebrovascular is likely the site of major dysfunction in the TBI model. (C) Microscopy of DHE staining (red) in the injured cortex; scale bar = 200 μm showing the prominent fluorescence in vascular structures (long thin structures). DAF-2DA levels (indicative of NO levels) are shown in (D) for the brain parenchyma (cortex) and (E) blood vessels of sham surgery rats or rats with TBI + hypotension with vehicle or PEG-HCCs treatment. (F) Microscopy of DAF-2DA staining (green) in the injured cortex. NO-related fluorescence was reduced following TBI and restored to levels comparable to the sham-treated animals. NO is important for vasodilation and increased CBF, therefore, increased DAF-2DA staining with PEG-HCC treatment correlates well with the improved CBF from Figure 3. Both DHE and DAF-2DA fluorescence was normalized in each animal to the contralateral cortex to account for minor differences in dye administration and circulation times. For all data in this Figure, error bars are S.E.M. D'Agostino-Pearson normality test was used on data and no groups had a p-value <0.05. ANOVA with Bonferroni post test was used to calculate statistics. * p-value < 0.05; ** p-value < 0.01; *** p-value < 0.001. N=5-6 animals/group with 10 brain sections analyzed per animal. Scale bar = 200 μm.
Similar articles
- Effects of hypertonic arginine on cerebral blood flow and intracranial pressure after traumatic brain injury combined with hemorrhagic hypotension.
Prough DS, Kramer GC, Uchida T, Stephenson RT, Hellmich HL, Dewitt DS. Prough DS, et al. Shock. 2006 Sep;26(3):290-5. doi: 10.1097/01.shk.0000225405.66693.49. Shock. 2006. PMID: 16912655 - SNP improves cerebral hemodynamics during normotension but fails to prevent sex dependent impaired cerebral autoregulation during hypotension after brain injury.
Armstead WM, Kiessling JW, Kofke WA, Vavilala MS. Armstead WM, et al. Brain Res. 2010 May 12;1330:142-50. doi: 10.1016/j.brainres.2010.03.024. Epub 2010 Mar 16. Brain Res. 2010. PMID: 20298682 Free PMC article. - Design of poly(ethylene glycol)-functionalized hydrophilic carbon clusters for targeted therapy of cerebrovascular dysfunction in mild traumatic brain injury.
Marcano DC, Bitner BR, Berlin JM, Jarjour J, Lee JM, Jacob A, Fabian RH, Kent TA, Tour JM. Marcano DC, et al. J Neurotrauma. 2013 May 1;30(9):789-96. doi: 10.1089/neu.2011.2301. Epub 2012 Nov 5. J Neurotrauma. 2013. PMID: 22928502 - Chapter 5 cerebral perfusion pressure and intracranial pressure in traumatic brain injury.
Mitchell PH, Kirkness C, Blissitt PA. Mitchell PH, et al. Annu Rev Nurs Res. 2015;33:111-83. doi: 10.1891/0739-6686.33.111. Annu Rev Nurs Res. 2015. PMID: 25946385 Review.
Cited by
- Antioxidant efficacy of chitosan/graphene functionalized superparamagnetic iron oxide nanoparticles.
Hastak V, Bandi S, Kashyap S, Singh S, Luqman S, Lodhe M, Peshwe DR, Srivastav AK. Hastak V, et al. J Mater Sci Mater Med. 2018 Sep 29;29(10):154. doi: 10.1007/s10856-018-6163-0. J Mater Sci Mater Med. 2018. PMID: 30269256 - Nanostructures: a platform for brain repair and augmentation.
Vidu R, Rahman M, Mahmoudi M, Enachescu M, Poteca TD, Opris I. Vidu R, et al. Front Syst Neurosci. 2014 Jun 20;8:91. doi: 10.3389/fnsys.2014.00091. eCollection 2014. Front Syst Neurosci. 2014. PMID: 24999319 Free PMC article. Review. - Physical and electrical characterization of TexasPEG: An electrically conductive neuronal scaffold.
Sikkema WKA, Metzger AB, Wang T, Tour JM. Sikkema WKA, et al. Surg Neurol Int. 2017 May 26;8:84. doi: 10.4103/sni.sni_361_16. eCollection 2017. Surg Neurol Int. 2017. PMID: 28607818 Free PMC article. - Molybdenum Nanodots for Acute Lung Injury Therapy.
Yan J, Tang Z, Li Y, Wang H, Hsu JC, Shi M, Fu Z, Ji X, Cai W, Ni D, Qu J. Yan J, et al. ACS Nano. 2023 Dec 12;17(23):23872-23888. doi: 10.1021/acsnano.3c08147. Epub 2023 Nov 21. ACS Nano. 2023. PMID: 38084420 Free PMC article. - Ultrasmall Mixed Eu-Gd Oxide Nanoparticles for Multimodal Fluorescence and Magnetic Resonance Imaging of Passive Accumulation and Retention in TBI.
Bony BA, Miller HA, Tarudji AW, Gee CC, Sarella A, Nichols MG, Kievit FM. Bony BA, et al. ACS Omega. 2020 Jun 23;5(26):16220-16227. doi: 10.1021/acsomega.0c01890. eCollection 2020 Jul 7. ACS Omega. 2020. PMID: 32656444 Free PMC article.
References
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral Autoregulation. Cerebrovasc. Brain. Metab. Rev. 1990;2:161–192. - PubMed
- DeWitt DS, Prough DS. Traumatic Cerebral Vascular Injury: the Effects of Concussive Brain Injury on the Cerebral Vasculature. J. Neurotrauma. 2003;20:795–825. - PubMed
- Butcher I, Maas AI, Lu J, Marmarou A, Murray GD, Mushkudiani NA, McHugh GS, Steyerberg EW. Prognostic Value of Admission Blood Pressure in Traumatic Brain Injury: Results from the IMPACT Study. J. Neurotrauma. 2007;24:294–302. - PubMed
- Kontos HA, Wei EP. Superoxide Production in Experimental Brain Injury. J. Neurosurg. 1986;64:803–807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL007676/HL/NHLBI NIH HHS/United States
- S10RR024574/RR/NCRR NIH HHS/United States
- R01 HL095586/HL/NHLBI NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- P30 DK079638/DK/NIDDK NIH HHS/United States
- NIH R01 HL095586/HL/NHLBI NIH HHS/United States
- S10 RR024574/RR/NCRR NIH HHS/United States
- P30 AI036211/AI/NIAID NIH HHS/United States
- NCI P30CA125123/CA/NCI NIH HHS/United States
- NIAID AI036211/PHS HHS/United States
- P30DK079638-02/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical