Withdrawal from cocaine self-administration alters NMDA receptor-mediated Ca2+ entry in nucleus accumbens dendritic spines - PubMed (original) (raw)
Withdrawal from cocaine self-administration alters NMDA receptor-mediated Ca2+ entry in nucleus accumbens dendritic spines
Carrie R Ferrario et al. PLoS One. 2012.
Abstract
We previously showed that the time-dependent intensification ("incubation") of cue-induced cocaine seeking after withdrawal from extended-access cocaine self-administration is accompanied by accumulation of Ca(2+)-permeable AMPA receptors (CP-AMPARs) in the rat nucleus accumbens (NAc). These results suggest an enduring change in Ca(2+) signaling in NAc dendritic spines. The purpose of the present study was to determine if Ca(2+) signaling via NMDA receptors (NMDARs) is also altered after incubation. Rats self-administered cocaine or saline for 10 days (6 h/day). After 45-47 days of withdrawal, NMDAR-mediated Ca(2+) entry elicited by glutamate uncaging was monitored in individual NAc dendritic spines. NMDAR currents were simultaneously recorded using whole cell patch clamp recordings. We also measured NMDAR subunit levels in a postsynaptic density (PSD) fraction prepared from the NAc of identically treated rats. NMDAR currents did not differ between groups, but a smaller percentage of spines in the cocaine group responded to glutamate uncaging with NMDAR-mediated Ca(2+) entry. No significant group differences in NMDAR subunit protein levels were found. The decrease in the proportion of spines showing NMDAR-mediated Ca(2+) entry suggests that NAc neurons in the cocaine group contain more spines which lack NMDARs (non-responding spines). The fact that cocaine and saline groups did not differ in NMDAR currents or NMDAR subunit levels suggests that the number of NMDARs on responding spines is not significantly altered by cocaine exposure. These findings are discussed in light of increases in dendritic spine density in the NAc observed after withdrawal from repeated cocaine exposure.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
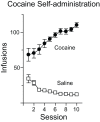
Figure 1. Self-administration behavior.
Mean number of infusions (± SEM) taken during each 6 hour self-administration session for saline (open squares) or cocaine (closed circles) groups.
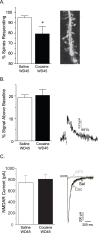
Figure 2. The percentage of spines exhibiting NMDAR-mediated Ca2+ entry is decreased after 45 days of withdrawal from cocaine self-administration.
A) Left: The percent of dendritic spines showing a NMDAR-mediated Ca2+ response upon photolysis of caged glutamate is significantly reduced in the cocaine group compared to the saline group. Right: Representative 2–photon image of a fura–2 filled dendrite from a MSN in the NAc core. B) Left: In spines that did exhibit an NMDAR-mediated Ca2+ response, the relative magnitude of the Ca2+ response did not differ between saline and cocaine groups. Right: Representative NMDAR-evoked Ca2+ transient from a MSN spine from the saline group. C) Left: The peak amplitude of the NMDAR-evoked whole cell currents did not differ between saline and cocaine groups. Right: Representative traces of NMDAR currents in MSN from saline (black) and cocaine (dark gray) groups. NMDAR currents in both groups were completely blocked by addition of APV (light gray trace shows APV blockade in a saline-treated animal). A 20 ms UV flash in the absence of caged MNI-glutamate did not generate a current response (data not shown). Data are presented as mean (± SEM). *p<0.05.
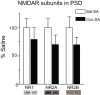
Figure 3. Expression of NMDAR subunits in the postsynaptic density (PSD) fraction after 45 days of withdrawal from cocaine or saline self-administration.
Data are presented as mean (± SEM) expressed as percent of saline controls. NR1, NR2A and NR2B protein levels in the cocaine group were slightly decreased on WD45 compared to the saline group, though this did not reach statistical significance. Representative blots are shown below each bar.
Similar articles
- GluN3-Containing NMDA Receptors in the Rat Nucleus Accumbens Core Contribute to Incubation of Cocaine Craving.
Christian DT, Stefanik MT, Bean LA, Loweth JA, Wunsch AM, Funke JR, Briggs CA, Lyons J, Neal D, Milovanovic M, D'Souza GX, Stutzmann GE, Nicholson DA, Tseng KY, Wolf ME. Christian DT, et al. J Neurosci. 2021 Sep 29;41(39):8262-8277. doi: 10.1523/JNEUROSCI.0406-21.2021. Epub 2021 Aug 19. J Neurosci. 2021. PMID: 34413203 Free PMC article. - Dynamic Alterations of Rat Nucleus Accumbens Dendritic Spines over 2 Months of Abstinence from Extended-Access Cocaine Self-Administration.
Christian DT, Wang X, Chen EL, Sehgal LK, Ghassemlou MN, Miao JJ, Estepanian D, Araghi CH, Stutzmann GE, Wolf ME. Christian DT, et al. Neuropsychopharmacology. 2017 Feb;42(3):748-756. doi: 10.1038/npp.2016.168. Epub 2016 Aug 24. Neuropsychopharmacology. 2017. PMID: 27555380 Free PMC article. - Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca²⁺-permeable AMPA receptors during the incubation of cocaine craving.
Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galiñanes GL, Heng LJ, Tseng KY, Wolf ME. Ferrario CR, et al. Neuropharmacology. 2011 Dec;61(7):1141-51. doi: 10.1016/j.neuropharm.2011.01.021. Epub 2011 Jan 27. Neuropharmacology. 2011. PMID: 21276808 Free PMC article. - Cocaine-induced metaplasticity in the nucleus accumbens: silent synapse and beyond.
Lee BR, Dong Y. Lee BR, et al. Neuropharmacology. 2011 Dec;61(7):1060-9. doi: 10.1016/j.neuropharm.2010.12.033. Epub 2011 Jan 11. Neuropharmacology. 2011. PMID: 21232547 Free PMC article. Review. - Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration.
Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Self DW, et al. Learn Mem. 2004 Sep-Oct;11(5):648-57. doi: 10.1101/lm.81404. Learn Mem. 2004. PMID: 15466321 Free PMC article. Review.
Cited by
- Retinoic acid-mediated homeostatic plasticity drives cell type-specific CP-AMPAR accumulation in nucleus accumbens core and incubation of cocaine craving.
Hwang EK, Wunsch AM, Wolf ME. Hwang EK, et al. bioRxiv [Preprint]. 2024 Sep 14:2024.09.12.611703. doi: 10.1101/2024.09.12.611703. bioRxiv. 2024. PMID: 39314388 Free PMC article. Preprint. - Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why?
Wolf ME, Tseng KY. Wolf ME, et al. Front Mol Neurosci. 2012 Jun 27;5:72. doi: 10.3389/fnmol.2012.00072. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22754497 Free PMC article. - Using Kalirin conditional knockout mice to distinguish its role in dopamine receptor mediated behaviors.
LaRese TP, Yan Y, Eipper BA, Mains RE. LaRese TP, et al. BMC Neurosci. 2017 May 23;18(1):45. doi: 10.1186/s12868-017-0363-2. BMC Neurosci. 2017. PMID: 28535798 Free PMC article. - Differential striatal spine pathology in Parkinson's disease and cocaine addiction: a key role of dopamine?
Villalba RM, Smith Y. Villalba RM, et al. Neuroscience. 2013 Oct 22;251:2-20. doi: 10.1016/j.neuroscience.2013.07.011. Epub 2013 Jul 16. Neuroscience. 2013. PMID: 23867772 Free PMC article. Review. - Time-dependent changes in nicotine behavioral responsivity during early withdrawal from chronic cocaine administration and attenuation of cocaine sensitization by mecamylamine.
Szabo ST, Fowler JC, Froeliger B, Lee TH. Szabo ST, et al. Behav Brain Res. 2014 Apr 1;262:42-6. doi: 10.1016/j.bbr.2013.12.051. Epub 2014 Jan 7. Behav Brain Res. 2014. PMID: 24412684 Free PMC article.
References
- Holtmaat A, Svoboda K (2009) Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10: 647–658. - PubMed
- Robinson TE, Kolb B (2004) Structural plasticity associated with exposure to drugs of abuse. Neuropharmacol 47: 33–46. - PubMed
- Robinson TE, Kolb B (1999) Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci 11: 1598–1604. - PubMed
- Robinson TE, Gorny G, Mitton E, Kolb B (2001) Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse 39: 257–266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 DA024502/DA/NIDA NIH HHS/United States
- DA024502/DA/NIDA NIH HHS/United States
- DA015835/DA/NIDA NIH HHS/United States
- K05 DA029099/DA/NIDA NIH HHS/United States
- R01 DA015835/DA/NIDA NIH HHS/United States
- R37 DA015835/DA/NIDA NIH HHS/United States
- DA029099/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous