An essential role for histone deacetylase 4 in synaptic plasticity and memory formation - PubMed (original) (raw)
An essential role for histone deacetylase 4 in synaptic plasticity and memory formation
Mi-Sung Kim et al. J Neurosci. 2012.
Abstract
Histone deacetylases (HDACs), a family of enzymes involved in epigenetic regulation, have been implicated in the control of synaptic plasticity, as well as learning and memory. Previous work has demonstrated administration of pharmacological HDAC inhibitors, primarily those targeted to class I HDACs, enhance learning and memory as well as long-term potentiation. However, a detailed understanding of the role of class II HDACs in these processes remains elusive. Here, we show that selective loss of Hdac4 in brain results in impairments in hippocampal-dependent learning and memory and long-term synaptic plasticity. In contrast, loss of Hdac5 does not impact learning and memory demonstrating unique roles in brain for individual class II HDACs. These findings suggest that HDAC4 is a crucial positive regulator of learning and memory, both behaviorally and at the cellular level, and that inhibition of Hdac4 activity may have unexpected detrimental effects to these processes.
Figures
Figure 1.
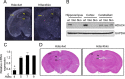
Generation of forebrain-specific Hdac4bko mice. A, Detection of Hdac4 transcripts by in situ hybridization in coronal sections from Hdac4wt and Hdac4bko mice at 4 months of age. B, Western blots of protein from brain lysates showed deletion of HDAC4 in the cortex and hippocampus, but not the cerebellum of Hdac4bko mice. GAPDH protein was used as a loading control. C, Expression levels of class II HDAC mRNAs (Hdac4, Hdac5, Hdac7, and Hdac9) in the hippocampus of Hdac4bko mice compared with Hdac4wt, as determined by quantitative RT-PCR. Error bars indicate ± SEM. *p < 0.05. D, Hematoxylin and eosin staining showed no obvious changes in brain morphology between 4-month-old Hdac4bko and Hdac4wt mice. CTX, Cortex; DG, dentate gyrus; Hp, hippocampus.
Figure 2.
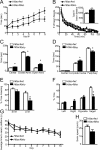
Hdac4bko mice show behavioral abnormalities including impaired memory formation. A, The Hdac4bko mice were tested on the rotarod for motor coordination. Based on repeated measures with mixed-model analysis, there were significant group effects between Hdac4bko and Hdac4wt mice (F(1,39) = 4.69; value of p = 0.0366) and trial effect (F(7,272) = 23.59; value of p < 0.0001). B, Hdac4bko mice display an increase in locomotor activity, as assessed by an increase in the number of beam breaks over 120 min. The Hdac4bko had a significant increase in the total number of beam breaks over 120 min compared with Hdac4wt mice (inset). Total locomotor activity was assessed for 120 min, and a significant total ambulation difference between the Hdac4bko and Hdac4wt mice was detected, demonstrating that the Hdac4bko mice were significantly hyperactive compared with littermate controls (*p < 0.05; inset). To better delineate the alteration in locomotor activity, the data were analyzed in 5 min increments. Based on repeated measures with mixed-model analysis, there were significant group effects between Hdac4bko and Hdac4wt mice (F(1,39) = 6.18; value of p = 0.0173) and time effect (F(23,897) = 109.44; value of p < 0.0001) further demonstrating the hyperactivity of the Hdac4bko mice. C, Hdac4bko show a significant increase in time spent in the center of the maze as well as the open arms, and a significant decrease in time spent in the closed arms (*p < 0.05; Hdac4wt, n = 18; Hdac4bko, n = 23). D, Hdac4bko were less anxious than littermate Hdac4wt mice in the open-field test. The Hdac4bko mice spent significantly more time in the complete center and less time in the periphery compared with Hdac4wt mice (*p < 0.05; Hdac4wt, n = 18; Hdac4bko, n = 23). E, The time spent freezing 90 min and 24 h after training was presented to examine STM and LTM, respectively, in the fear-conditioning paradigm. Results indicate a statistically significant decrease in LTM formation but not STM in the Hdac4bko compared with Hdac4wt mice (*p < 0.05; Hdac4wt, n = 8; Hdac4bko, n = 13). F, Hdac4bko mice are impaired in spatial learning in the Morris water maze. A probe trial was performed on day 11. Hdac4bko mice spent a similar amount of time in the four quadrants, while the Hdac4wt mice showed a preference for the target quadrant and spent significantly more time in the target quadrant (*p < 0.05; Hdac4wt, n = 11; Hdac4bko, n = 11). G, Hdac4bko mice show no differences from Hdac4wt mice in the average time to reach the platform during the 10 d training (*p < 0.05; Hdac4wt, n = 11; Hdac4bko, n = 11). H, Hdac4wt mice and Hdac4bko mice have similar swim speeds, and there were no significant differences in average swim velocity between the two groups (*p < 0.05; Hdac4wt, n = 11; Hdac4bko, n = 11). For all experiments, error bars show SEM.
Figure 3.
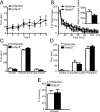
_Hdac5_−/− mice have normal behavior including memory formation. A, _Hdac5_−/− mice have normal motor coordination, staying on the rotarod as well as littermate Hdac5wt. The _Hdac5_−/− mice were indistinguishable from Hdac5wt mice in motor coordination as assessed by the rotarod test, as repeated measures with mixed-model analysis revealed no significant group effect (F(1,6) = 0.06; value of p = 0.8109). B, The _Hdac5_−/− mice were examined for alterations in total locomotor activity for 120 min, and using Student's t test analysis, there was no significant difference between _Hdac5_−/− and Hdac5wt mice. We also assessed ambulation in 5 min increments and, based on repeated measures with mixed-model analysis, found no significant group effect between _Hdac5_−/− and Hdac5wt mice (F(1,6) = 5.87; value of p = 0.0517), while there is a significant time effect (F(23,138) = 38.20; value of p < 0.0001). C, In the elevated plus maze test, _Hdac5_−/− mice show no significant difference in time spent in the center of the maze, closed arms, or open arms compared with Hdac5wt mice. D, In the open-field test, the _Hdac5_−/− mice spent a similar amount of time in the complete center and periphery compared with Hdac5wt mice. E, _Hdac5_−/− mice show normal context-dependent fear conditioning. The time spent freezing 24 h after training in a one-trial fear-conditioning paradigm was assessed. Results indicate no difference in fear memory in the _Hdac5_−/− mice compared with Hdac5wt mice (_Hdac5_−/−, n = 7; Hdac5wt, n = 8).
Figure 4.
Impaired hippocampal plasticity in Hdac4bko mice. A, Hdac4bko mice display impaired LTP at Schaffer collateral/CA1 pyramidal cell synapses compared with Hdac4wt animals as assessed by a two-way ANOVA with repeated measures (*p < 0.05; Hdac4wt, n = 7; Hdac4bko, n = 8). B, The averaged fEPSP plotted against presynaptic volley amplitude shows no significant difference in input–output function of CA1-fEPSPs in Hdac4bko mice compared with Hdac4wt controls (Hdac4wt, n = 7; Hdac4bko, n = 8). Input–output slopes were fit by linear regression and between group slopes were subsequently compared using unpaired t tests. C, The averaged paired-pulse facilitation for Hdac4wt and Hdac4bko slices at various intervals at SC-CA1 synapses. Using Student's t test, significant decreases in Hdac4bko mice were observed at the first three interstimulus interval data (20, 30, and 50 ms) (*p < 0.05; Hdac4wt, n = 7; Hdac4bko, n = 8).
Figure 5.
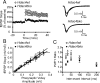
Deletion of Hdac4 or Hdac5 in hippocampal neurons in culture does not perturb basal synaptic transmission. A, Representative recordings of miniature excitatory events recorded in 1 μ
m
tetrodotoxin and 50 μ
m
picrotoxin from homozygous Hdac4loxP/loxP neurons infected with either high-titer lentivirus expressing GFP and CRE-GFP. B, C, Bar graph depicts that mEPSC frequency and amplitudes remain unchanged upon Hdac4 knockdown. The numbers on the bars indicate the number of experiments. D, Representative recordings of miniature excitatory events recorded from _Hdac5_−/− and Hdac5wt neurons. E, F, Bar graphs reveal no changes in mEPSC frequency or amplitudes upon Hdac5 deletion. All the recordings were made on 14–17 DIV hippocampal neuronal cultures. Error bars indicate ±SEM.
Similar articles
- Vezatin is essential for dendritic spine morphogenesis and functional synaptic maturation.
Danglot L, Freret T, Le Roux N, Narboux Nême N, Burgo A, Hyenne V, Roumier A, Contremoulins V, Dauphin F, Bizot JC, Vodjdani G, Gaspar P, Boulouard M, Poncer JC, Galli T, Simmler MC. Danglot L, et al. J Neurosci. 2012 Jun 27;32(26):9007-22. doi: 10.1523/JNEUROSCI.3084-11.2012. J Neurosci. 2012. PMID: 22745500 Free PMC article. - 14-3-3 proteins are required for hippocampal long-term potentiation and associative learning and memory.
Qiao H, Foote M, Graham K, Wu Y, Zhou Y. Qiao H, et al. J Neurosci. 2014 Apr 2;34(14):4801-8. doi: 10.1523/JNEUROSCI.4393-13.2014. J Neurosci. 2014. PMID: 24695700 Free PMC article. - Activity-dependent regulation of synaptic strength by PSD-95 in CA1 neurons.
Zhang P, Lisman JE. Zhang P, et al. J Neurophysiol. 2012 Feb;107(4):1058-66. doi: 10.1152/jn.00526.2011. Epub 2011 Nov 23. J Neurophysiol. 2012. PMID: 22114157 Free PMC article. - Protease-activated receptor-1 modulates hippocampal memory formation and synaptic plasticity.
Almonte AG, Qadri LH, Sultan FA, Watson JA, Mount DJ, Rumbaugh G, Sweatt JD. Almonte AG, et al. J Neurochem. 2013 Jan;124(1):109-22. doi: 10.1111/jnc.12075. Epub 2012 Nov 21. J Neurochem. 2013. PMID: 23113835 Free PMC article. - Histone deacetylases govern cellular mechanisms underlying behavioral and synaptic plasticity in the developing and adult brain.
Morris MJ, Karra AS, Monteggia LM. Morris MJ, et al. Behav Pharmacol. 2010 Sep;21(5-6):409-19. doi: 10.1097/FBP.0b013e32833c20c0. Behav Pharmacol. 2010. PMID: 20555253 Free PMC article. Review.
Cited by
- The dark side of HDAC inhibition in ALS.
Boutillier AL, Tzeplaeff L, Dupuis L. Boutillier AL, et al. EBioMedicine. 2019 Mar;41:38-39. doi: 10.1016/j.ebiom.2019.02.039. Epub 2019 Feb 23. EBioMedicine. 2019. PMID: 30803935 Free PMC article. No abstract available. - Behavioral Alterations in Mice Carrying Homozygous HDAC4 A778T Missense Mutation Associated With Eating Disorder.
Davis KC, Saito K, Rodeghiero SR, Toth BA, Lutter M, Cui H. Davis KC, et al. Front Neurosci. 2020 Feb 21;14:139. doi: 10.3389/fnins.2020.00139. eCollection 2020. Front Neurosci. 2020. PMID: 32153359 Free PMC article. - Evaluation of Class IIa Histone Deacetylases Expression and In Vivo Epigenetic Imaging in a Transgenic Mouse Model of Alzheimer's Disease.
Chen YA, Lu CH, Ke CC, Chiu SJ, Chang CW, Yang BH, Gelovani JG, Liu RS. Chen YA, et al. Int J Mol Sci. 2021 Aug 11;22(16):8633. doi: 10.3390/ijms22168633. Int J Mol Sci. 2021. PMID: 34445342 Free PMC article. - Understanding the Role of Histone Deacetylase and their Inhibitors in Neurodegenerative Disorders: Current Targets and Future Perspective.
Kumar V, Kundu S, Singh A, Singh S. Kumar V, et al. Curr Neuropharmacol. 2022;20(1):158-178. doi: 10.2174/1570159X19666210609160017. Curr Neuropharmacol. 2022. PMID: 34151764 Free PMC article. Review. - The Molecular Basis of Depression: Implications of Sex-Related Differences in Epigenetic Regulation.
Kawatake-Kuno A, Murai T, Uchida S. Kawatake-Kuno A, et al. Front Mol Neurosci. 2021 Jul 1;14:708004. doi: 10.3389/fnmol.2021.708004. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34276306 Free PMC article.
References
- Alarcón JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. - PubMed
- Arnold MA, Kim Y, Czubryt MP, Phan D, McAnally J, Qi X, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell. 2007;12:377–389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH066198/MH/NIMH NIH HHS/United States
- MH066198/MH/NIMH NIH HHS/United States
- R01 MH081060/MH/NIMH NIH HHS/United States
- MH081060/MH/NIMH NIH HHS/United States
- R56 MH081060/MH/NIMH NIH HHS/United States
- R01 HL093039/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases