Prolonged injury and altered lung function after ozone inhalation in mice with chronic lung inflammation - PubMed (original) (raw)
Prolonged injury and altered lung function after ozone inhalation in mice with chronic lung inflammation
Angela M Groves et al. Am J Respir Cell Mol Biol. 2012 Dec.
Abstract
Surfactant protein-D (Sftpd) is a pulmonary collectin important in down-regulating macrophage inflammatory responses. In these experiments, we analyzed the effects of chronic macrophage inflammation attributable to loss of Sftpd on the persistence of ozone-induced injury, macrophage activation, and altered functioning in the lung. Wild-type (Sftpd(+/+)) and Sftpd(-/-) mice (aged 8 wk) were exposed to air or ozone (0.8 parts per million, 3 h). Bronchoalveolar lavage (BAL) fluid and tissue were collected 72 hours later. In Sftpd(-/-) mice, but not Sftpd(+/+) mice, increased BAL protein and nitrogen oxides were observed after ozone inhalation, indicating prolonged lung injury and oxidative stress. Increased numbers of macrophages were also present in BAL fluid and in histologic sections from Sftpd(-/-) mice. These cells were enlarged and foamy, suggesting that they were activated. This conclusion was supported by findings of increased BAL chemotactic activity, and increased expression of inducible nitric oxide synthase in lung macrophages. In both Sftpd(+/+) and Sftpd(-/-) mice, inhalation of ozone was associated with functional alterations in the lung. Although these alterations were limited to central airway mechanics in Sftpd(+/+) mice, both central airway and parenchymal mechanics were modified by ozone exposure in Sftpd(-/-) mice. The most notable changes were evident in resistance and elastance spectra and baseline lung function, and in lung responsiveness to changes in positive end-expiratory pressure. These data demonstrate that a loss of Sftpd is associated with prolonged lung injury, oxidative stress, and macrophage accumulation and activation in response to ozone, and with more extensive functional changes consistent with the loss of parenchymal integrity.
Figures
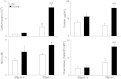
Figure 1.
Effects of ozone inhalation on lung inflammation and injury. Bronchoalveolar lavage (BAL) was collected 72 hours after exposure of Sftpd+/+ and Sftpd−/− mice to air or ozone and analyzed for cell, protein, and nitrogen oxides (NOx) content, and for chemotactic activity. BAL chemotactic activity was assessed using the RAW 264.7 murine macrophage cell line (American Type Culture Collection, Manassas, VA), as previously described (15). Hanks’ balanced salt solution was used as the negative control. Chemotaxis data are expressed as the average number of migrated cells per 20 high-power fields (HPF). The value range for these experiments was 0–30 migrated cells per high power field. Each bar represents the mean ± SEM of three experiments (n = 4–9 mice/treatment group/experiment; n = 12–27 mice/group total). Data were analyzed by two-way ANOVA. aSignificantly different (P ≤ 0.05) from air-exposed control mice. bSignificantly different (P ≤ 0.05) from Sftpd+/+ mice. Sftpd, surfactant protein–D.
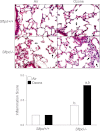
Figure 2.
Effects of ozone inhalation on lung structure and inflammation score. Top: Lung sections, prepared 72 hours after exposure of Sftpd+/+ and Sftpd−/− mice to air or ozone, were stained with hematoxylin and eosin. Arrows indicate alveolar macrophages. Original magnification, ×600. Bottom: Slides were scored blindly by two independent observers for severity of inflammation on a scale of 0–5 (0, no inflammation; 5, severe inflammation). Each bar represents the median of three experiments (n = 4–9 mice/treatment group/experiment; n = 12–27 mice/group total). Significance was assessed by ANOVA, based on ranks. aSignificantly different (P ≤ 0.05) from air-exposed control mice. bSignificantly different (P ≤ 0.05) from Sftpd+/+ mice.
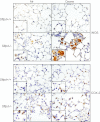
Figure 3.
Effects of ozone inhalation on lung expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). Lung sections, prepared 72 hours after exposure of Sftpd+/+ and Sftpd−/− mice to air or ozone, were stained with a 1:100 dilution of antibody to iNOS (above), a 1:1,000 dilution of antibody to COX-2 (below), or IgG control, followed by a 1:200 dilution of biotinylated secondary antibody. Binding was visualized using a peroxidase substrate DAB kit (Vector Laboratories, Burlingame, CA). Insets show iNOS-positive or COX-2–positive cells. One representative section is shown from three separate experiments (n = 3 mice/treatment group/experiment). Original magnification, ×600; inset, ×1,000.
Figure 4.
Effects of ozone inhalation on central airway resistance (Rn) and static compliance (Cst) in Sftpd+/+ and Sftpd−/− mice. Lung function was measured 72 hours after exposure of Sftpd+/+ (circles) and Sftpd−/− (squares) mice to air (open symbols) or ozone (solid symbols). Lungs were subjected to increasing positive end-expiratory pressure (PEEP). Top row: For Rn, impedance spectra were measured using the forced oscillation technique. Results were analyzed using the constant phase model. Bottom row: Cst was calculated from pressure–volume loops. Measurements were performed in triplicate at each PEEP. For each sample at each PEEP, Rn and Cst values were normalized to PEEP = 3, the physiological pressure for mice. Each point represents the mean ± SEM of three experiments (n = 4–6 mice/treatment group/experiment; n = 12–27 mice/group total). Data were analyzed by two-way ANOVA and a nonpaired, two-tailed Student t test. *Significantly different (P ≤ 0.05) from air-exposed or Sftpd+/+ control mice.
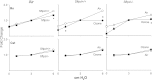
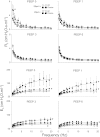
Figure 5.
Effects of ozone inhalation on resistance spectra curves (RL) and elastance spectra curves (EL) in Sftpd+/+ and Sftpd−/− mice. Lung function was measured 72 hours after exposure of Sftpd+/+ and Sftpd−/− mice to air or ozone. Lungs were subjected to increasing PEEP. Impedance spectra were generated using the forced oscillation technique. Resistance and elastance spectra were then derived from input impedance data. Measurements were performed in triplicate at each PEEP. Each point represents the mean ± SEM of three experiments (n = 4–6 mice/treatment group/experiment; n = 12–27 mice/group total). Data were analyzed by nonlinear regression. *Significantly different (P ≤ 0.05) from air-exposed control mice.
Similar articles
- Age-related increases in ozone-induced injury and altered pulmonary mechanics in mice with progressive lung inflammation.
Groves AM, Gow AJ, Massa CB, Hall L, Laskin JD, Laskin DL. Groves AM, et al. Am J Physiol Lung Cell Mol Physiol. 2013 Oct 15;305(8):L555-68. doi: 10.1152/ajplung.00027.2013. Epub 2013 Aug 30. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23997172 Free PMC article. - Regulation of ozone-induced lung inflammation and injury by the β-galactoside-binding lectin galectin-3.
Sunil VR, Francis M, Vayas KN, Cervelli JA, Choi H, Laskin JD, Laskin DL. Sunil VR, et al. Toxicol Appl Pharmacol. 2015 Apr 15;284(2):236-45. doi: 10.1016/j.taap.2015.02.002. Epub 2015 Feb 25. Toxicol Appl Pharmacol. 2015. PMID: 25724551 Free PMC article. - Acute chlorine gas exposure produces transient inflammation and a progressive alteration in surfactant composition with accompanying mechanical dysfunction.
Massa CB, Scott P, Abramova E, Gardner C, Laskin DL, Gow AJ. Massa CB, et al. Toxicol Appl Pharmacol. 2014 Jul 1;278(1):53-64. doi: 10.1016/j.taap.2014.02.006. Epub 2014 Feb 25. Toxicol Appl Pharmacol. 2014. PMID: 24582687 Free PMC article. - Nitric oxide and peroxynitrite in ozone-induced lung injury.
Laskin DL, Fakhrzadeh L, Laskin JD. Laskin DL, et al. Adv Exp Med Biol. 2001;500:183-90. doi: 10.1007/978-1-4615-0667-6_24. Adv Exp Med Biol. 2001. PMID: 11764933 Review. - Does tissue imprinting restrict macrophage plasticity?
Guilliams M, Svedberg FR. Guilliams M, et al. Nat Immunol. 2021 Feb;22(2):118-127. doi: 10.1038/s41590-020-00849-2. Epub 2021 Jan 18. Nat Immunol. 2021. PMID: 33462453 Review.
Cited by
- Single-Nuclei Characterization of Lacrimal Gland in Scopolamine-Induced Dry Eye Disease.
Tang Y, Dou S, Wei C, Sun Z, Sun D, Zhou Q, Xie L. Tang Y, et al. Invest Ophthalmol Vis Sci. 2024 Apr 1;65(4):46. doi: 10.1167/iovs.65.4.46. Invest Ophthalmol Vis Sci. 2024. PMID: 38687491 Free PMC article. - The Oxygen-Ozone Adjunct Medical Treatment According to the Protocols from the Italian Scientific Society of Oxygen-Ozone Therapy: How Ozone Applications in the Blood Can Influence Clinical Therapy Success via the Modulation of Cell Biology and Immunity.
Chirumbolo S, Valdenassi L, Tirelli U, Ricevuti G, Pandolfi S, Vaiano F, Galoforo A, Loprete F, Simonetti V, Chierchia M, Bellardi D, Richelmi T, Franzini M. Chirumbolo S, et al. Biology (Basel). 2023 Dec 11;12(12):1512. doi: 10.3390/biology12121512. Biology (Basel). 2023. PMID: 38132338 Free PMC article. - Role of PPARγ in dyslipidemia and altered pulmonary functioning in mice following ozone exposure.
Smith LC, Gow AJ, Abramova E, Vayas K, Guo C, Noto J, Lyman J, Rodriquez J, Gelfand-Titiyevskiy B, Malcolm C, Laskin JD, Laskin DL. Smith LC, et al. Toxicol Sci. 2023 Jun 28;194(1):109-119. doi: 10.1093/toxsci/kfad048. Toxicol Sci. 2023. PMID: 37202362 Free PMC article. - Suppression of Lung Oxidative Stress, Inflammation, and Fibrosis following Nitrogen Mustard Exposure by the Selective Farnesoid X Receptor Agonist Obeticholic Acid.
Meshanni JA, Lee JM, Vayas KN, Sun R, Jiang C, Guo GL, Gow AJ, Laskin JD, Laskin DL. Meshanni JA, et al. J Pharmacol Exp Ther. 2024 Jan 17;388(2):586-595. doi: 10.1124/jpet.123.001557. J Pharmacol Exp Ther. 2024. PMID: 37188530 - Development and validation of an immune-related gene signature for prognosis in Lung adenocarcinoma.
Guo Z, Qi X, Li Z, Yang J, Sun Z, Li P, Chen M, Cao Y. Guo Z, et al. IET Syst Biol. 2023 Feb;17(1):27-38. doi: 10.1049/syb2.12057. Epub 2023 Feb 2. IET Syst Biol. 2023. PMID: 36728032 Free PMC article.
References
- Foster WM, Brown RH, Macri K, Mitchell CS. Bronchial reactivity of healthy subjects: 18–20 h postexposure to ozone. J Appl Physiol 2000;89:1804–1810 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA132624/CA/NCI NIH HHS/United States
- R01 HL086621/HL/NHLBI NIH HHS/United States
- T32 ES007148/ES/NIEHS NIH HHS/United States
- R01CA132624/CA/NCI NIH HHS/United States
- R01 GM034310/GM/NIGMS NIH HHS/United States
- R01HL086621/HL/NHLBI NIH HHS/United States
- U54 AR055073/AR/NIAMS NIH HHS/United States
- U54AR055073/AR/NIAMS NIH HHS/United States
- P30ES005022/ES/NIEHS NIH HHS/United States
- R01 ES004738/ES/NIEHS NIH HHS/United States
- P30 ES005022/ES/NIEHS NIH HHS/United States
- R01ES004738/ES/NIEHS NIH HHS/United States
- R01GM034310/GM/NIGMS NIH HHS/United States
- T32ES007148/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous