Storage-induced changes in erythrocyte membrane proteins promote recognition by autoantibodies - PubMed (original) (raw)
Storage-induced changes in erythrocyte membrane proteins promote recognition by autoantibodies
Sip Dinkla et al. PLoS One. 2012.
Abstract
Physiological erythrocyte removal is associated with a selective increase in expression of neoantigens on erythrocytes and their vesicles, and subsequent autologous antibody binding and phagocytosis. Chronic erythrocyte transfusion often leads to immunization and the formation of alloantibodies and autoantibodies. We investigated whether erythrocyte storage leads to the increased expression of non-physiological antigens. Immunoprecipitations were performed with erythrocytes and vesicles from blood bank erythrocyte concentrates of increasing storage periods, using patient plasma containing erythrocyte autoantibodies. Immunoprecipitate composition was identified using proteomics. Patient plasma antibody binding increased with erythrocyte storage time, while the opposite was observed for healthy volunteer plasma, showing that pathology-associated antigenicity changes during erythrocyte storage. Several membrane proteins were identified as candidate antigens. The protein complexes that were precipitated by the patient antibodies in erythrocytes were different from the ones in the vesicles formed during erythrocyte storage, indicating that the storage-associated vesicles have a different immunization potential. Soluble immune mediators including complement factors were present in the patient plasma immunoprecipitates, but not in the allogeneic control immunoprecipitates. The results support the theory that disturbed erythrocyte aging during storage of erythrocyte concentrates contributes to transfusion-induced alloantibody and autoantibody formation.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
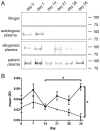
Figure 1. Erythrocyte autoantibody immunoprecipitation of erythrocytes sampled at regular time intervals during storage.
Analysis was performed by SDS-PAGE, followed by silver staining. (A) Protein patterns of precipitates obtained using Ringer, autologous plasma, and a representative example from one out of three allogeneic plasmas, and one out of six autoantibody-containing plasmas (patient No. 2). For the allogeneic controls, day 14 is missing. (B) Mean optical density (OD) of patient (•, solid line, N = 6 patients) and allogeneic control plasma (▴, dotted line, N = 3 volunteers) precipitations. Numbers indicate approximate molecular weight (kDa). Error bars represent standard error, *p<0.05.
Figure 2. Erythrocyte autoantibody immunoprecipitation of stored erythrocytes.
(A) Immunoprecipitation of 35 day old erythrocytes with erythrocyte autoantibody-containing patient plasma and allogeneic control plasma, using TX100 or RIPA extraction buffer and analyzed by SDS-PAGE under reducing or non-reducing conditions, followed by silver staining. A representative result (patient No. 2) from one out of three patient plasmas is shown. (B) Example of a silver stained gel of an immunoprecipitation of 35 day stored erythrocytes with plasma of patient No. 1. The same sample was used for Coomassie blue gel staining and subsequent proteomics analysis (Table 2). Gel slices which were excised for proteomic analyses are indicated as slices I and II (see also Table S1). Numbers indicate molecular weight (kDa). Heavy [H] and light [L] antibody chains are indicated by arrows.
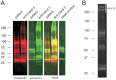
Figure 3. Erythrocyte autoantibody immunoprecipitation of biotinylated erythrocyte vesicles from a 35 day old transfusion unit.
(A) Immunoprecipitation with either plasma from patient No. 2, or a monoclonal antibody against band 3 (see Materials and Methods). Analysis was performed by SDS-PAGE, followed by detection of biotinylated membrane proteins (red, streptavidin) and band 3 (green, polyclonal rabbit antibody). A protein G bead control was included. (B) Example immunoprecipitation of biotinylated erythrocyte vesicles from a 35 day old transfusion unit using plasma from patient No. 1. Analysis was performed by SDS-PAGE, followed by detection of biotinylated membrane proteins using fluorochrome conjugated streptavidin. The same sample was used for Coomassie blue gel staining and subsequent proteomics analysis (Table 2). The gel slice which was excised for proteomic analysis is indicated as slice III (see also Table S1). Numbers indicate approximate molecular weight (kDa). Blots were analyzed using the Odyssey Infrared Imaging System.
Similar articles
- The proteome of red cell membranes and vesicles during storage in blood bank conditions.
Bosman GJ, Lasonder E, Luten M, Roerdinkholder-Stoelwinder B, Novotný VM, Bos H, De Grip WJ. Bosman GJ, et al. Transfusion. 2008 May;48(5):827-35. doi: 10.1111/j.1537-2995.2007.01630.x. Epub 2008 Mar 12. Transfusion. 2008. PMID: 18346024 - Comparative proteomics of erythrocyte aging in vivo and in vitro.
Bosman GJ, Lasonder E, Groenen-Döpp YA, Willekens FL, Werre JM, Novotný VM. Bosman GJ, et al. J Proteomics. 2010 Jan 3;73(3):396-402. doi: 10.1016/j.jprot.2009.07.010. Epub 2009 Aug 4. J Proteomics. 2010. PMID: 19660581 Review. - Erythrocyte ageing in vivo and in vitro: structural aspects and implications for transfusion.
Bosman GJ, Werre JM, Willekens FL, Novotný VM. Bosman GJ, et al. Transfus Med. 2008 Dec;18(6):335-47. doi: 10.1111/j.1365-3148.2008.00892.x. Transfus Med. 2008. PMID: 19140816 Review. - RBC-derived vesicles during storage: ultrastructure, protein composition, oxidation, and signaling components.
Kriebardis AG, Antonelou MH, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. Kriebardis AG, et al. Transfusion. 2008 Sep;48(9):1943-53. doi: 10.1111/j.1537-2995.2008.01794.x. Epub 2008 Jun 28. Transfusion. 2008. PMID: 18564399
Cited by
- Vesiculation of Red Blood Cells in the Blood Bank: A Multi-Omics Approach towards Identification of Causes and Consequences.
Freitas Leal JK, Lasonder E, Sharma V, Schiller J, Fanelli G, Rinalducci S, Brock R, Bosman G. Freitas Leal JK, et al. Proteomes. 2020 Mar 31;8(2):6. doi: 10.3390/proteomes8020006. Proteomes. 2020. PMID: 32244435 Free PMC article. - Age-Markers on the Red Blood Cell Surface and Erythrocyte Microparticles may Constitute a Multi-parametric Strategy for Detection of Autologous Blood Transfusion.
Biasini GM, Botrè F, de la Torre X, Donati F. Biasini GM, et al. Sports Med Open. 2023 Dec 1;9(1):113. doi: 10.1186/s40798-023-00662-9. Sports Med Open. 2023. PMID: 38038869 Free PMC article. - Survival of red blood cells after transfusion: processes and consequences.
Bosman GJ. Bosman GJ. Front Physiol. 2013 Dec 18;4:376. doi: 10.3389/fphys.2013.00376. Front Physiol. 2013. PMID: 24391593 Free PMC article. Review. - Red Blood Cell Homeostasis: Mechanisms and Effects of Microvesicle Generation in Health and Disease.
Leal JKF, Adjobo-Hermans MJW, Bosman GJCGM. Leal JKF, et al. Front Physiol. 2018 Jun 8;9:703. doi: 10.3389/fphys.2018.00703. eCollection 2018. Front Physiol. 2018. PMID: 29937736 Free PMC article. Review. - Impact of Storage Lesion on Post-transfusion Rise in Hemoglobin.
Sardar M, Shaikh N, Ansell J, Jacob A, Yada S, Kelly DB, Doraiswamy M, Khan WJ, Anwer F, Eng MH. Sardar M, et al. Cureus. 2018 Jul 9;10(7):e2952. doi: 10.7759/cureus.2952. Cureus. 2018. PMID: 30202679 Free PMC article.
References
- Bosman GJ, Werre JM, Willekens FL, Novotny VM (2008) Erythrocyte ageing in vivo and in vitro: structural aspects and implications for transfusion. Transfus Med 18: 335–347. - PubMed
- Willekens FL, Werre JM, Groenen-Dopp YA, Roerdinkholder-Stoelwinder B, de Pauw B, et al. (2008) Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol 141: 549–556. - PubMed
- Lang KS, Lang PA, Bauer C, Duranton C, Wieder T, et al. (2005) Mechanisms of suicidal erythrocyte death. Cell Physiol Biochem 15: 195–202. - PubMed
- Antonelou MH, Kriebardis AG, Stamoulis KE, Economou-Petersen E, Margaritis LH, et al. (2010) Red blood cell aging markers during storage in citrate-phosphate-dextrose-saline-adenine-glucose-mannitol. Transfusion 50: 376–389. - PubMed
- Messana I, Ferroni L, Misiti F, Girelli G, Pupella S, et al. (2000) Blood bank conditions and RBCs: the progressive loss of metabolic modulation. Transfusion 40: 353–360. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources