Cell polarity as a regulator of cancer cell behavior plasticity - PubMed (original) (raw)
Review
Cell polarity as a regulator of cancer cell behavior plasticity
Senthil K Muthuswamy et al. Annu Rev Cell Dev Biol. 2012.
Abstract
Cell polarization is an evolutionarily conserved process that facilitates asymmetric distribution of organelles and proteins and that is modified dynamically during physiological processes such as cell division, migration, and morphogenesis. The plasticity with which cells change their behavior and phenotype in response to cell intrinsic and extrinsic cues is an essential feature of normal physiology. In disease states such as cancer, cells lose their ability to behave normally in response to physiological cues. A molecular understanding of mechanisms that alter the behavior of cancer cells is limited. Cell polarity proteins are a recognized class of molecules that can receive and interpret both intrinsic and extrinsic signals to modulate cell behavior. In this review, we discuss how cell polarity proteins regulate a diverse array of biological processes and how they can contribute to alterations in the behavior of cancer cells.
Figures
Figure 1
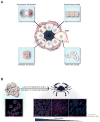
Cell polarity in normal epithelial organs and cancer. (a) Different types of polarity that are likely to regulate morphogenesis of epithelial structures. Planar cell polarity, used by epithelial cells to coordinate polarity between cells; apical-basal polarity, used by epithelial cells to asymmetrically distribute proteins along the apical-basal axis; front-rear polarity, used by migrating cells to maintain directionality; and mitotic spindle polarity, used for controlling position of daughter cells. (b, upper) Cancer is a consequence of both loss of normal growth control and disruption of normal cell behaviors indicated above. (b, lower) The immunostaining images of normal human breast and cancer with an apical marker (ZO-1) and a basolateral marker (E-cadherin). Normal tissue shows the presence of normal apical-basal polarity and overall organization of ducts with a layer of epithelial cells (tissue polarity); tumor tissue exhibits progressive loss of apical-basal and tissue polarity. Abbreviations: EMT, epithelial to mesenchymal transition
Figure 2
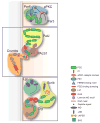
Cell polarity proteins in mammalian epithelia. Shown are the protein diagrams of the (a) subapically localized Par complex, (b) Crumbs complex, and (c) basolaterally localized Scribble complex and the protein interactions within the complexes, as reported previously. (a) In the Par complex, Par6 binds to aPKC through its N-terminal PB1 domain, and its PDZ domain binds to Par3. Par3 also interacts with aPKC through its C-terminal tail. (b) In the Crumbs complex, the cytoplasmic tail of the transmembrane protein Crumbs contains a PDZ-binding domain that interacts directly with the PDZ domain of PALS1. The interactions between PALS1 and PATJ involve the binding of the L27 domain of each protein. (c) Scribble associates indirectly with Dlg via a GUK holder protein. The interaction between Lgl and the rest of the complex is not yet clear. (d) The core PCP molecules distribute asymmetrically along the proximal-distal axis. Vang-like protein binds to Prickle (Pk) at the proximal end of the cell. The transmembrane protein Frizzled (Fzd) binds to Dgo and Dsh at the distal end of the cell. CELSR1 localizes to both ends to recruit VANGL (Vang) or Fzd to the membrane. Abbreviations: Par, partitioning defective; aPKC, atypical protein kinase C; Dlg, discs large; Lgl, lethal giant larvae; PATJ, Pals1 associated tight junction; PB1, Phox Bem 1; PDZ, Post synaptic density 95/Discs large/Zonula occludens-1; PCP, planar cell polarity; GUK, guanylate kinase.
Figure 3
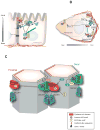
The central role of polarity protein complexes is retooled and rewired during cell polarization under different cellular contexts. The figure illustrates the changes in localization of polarity proteins and regulation of downstream targets during the transition between (a) apical-basal polarity and (b) front-rear polarity. Abbreviations: aPKC, atypical protein kinase C; Dlg, discs large; Lgl, lethal giant larvae; ECM, extracellular matrix; PATJ, Pals1 associated tight junction; GSK, glycogen synthase kinase, PItdIns, phosphatidylinositol; P, phosphorylation.
Similar articles
- Epithelial cell polarity determinant CRB3 in cancer development.
Li P, Mao X, Ren Y, Liu P. Li P, et al. Int J Biol Sci. 2015 Jan 1;11(1):31-7. doi: 10.7150/ijbs.10615. eCollection 2015. Int J Biol Sci. 2015. PMID: 25552927 Free PMC article. Review. - Par complex in cancer: a regulator of normal cell polarity joins the dark side.
Aranda V, Nolan ME, Muthuswamy SK. Aranda V, et al. Oncogene. 2008 Nov 24;27(55):6878-87. doi: 10.1038/onc.2008.340. Oncogene. 2008. PMID: 19029931 Free PMC article. Review. - Protein Complex Assemblies in Epithelial Cell Polarity and Asymmetric Cell Division.
Wen W, Zhang M. Wen W, et al. J Mol Biol. 2018 Sep 28;430(19):3504-3520. doi: 10.1016/j.jmb.2017.09.013. Epub 2017 Sep 27. J Mol Biol. 2018. PMID: 28963071 Review. - Scribble: A master scaffold in polarity, adhesion, synaptogenesis, and proliferation.
Bonello TT, Peifer M. Bonello TT, et al. J Cell Biol. 2019 Mar 4;218(3):742-756. doi: 10.1083/jcb.201810103. Epub 2018 Dec 31. J Cell Biol. 2019. PMID: 30598480 Free PMC article. Review. - Polarity as a physiological modulator of cell function.
Piroli ME, Blanchette JO, Jabbarzadeh E. Piroli ME, et al. Front Biosci (Landmark Ed). 2019 Jan 1;24(3):451-462. doi: 10.2741/4728. Front Biosci (Landmark Ed). 2019. PMID: 30468666 Free PMC article. Review.
Cited by
- TMEM25 is a Par3-binding protein that attenuates claudin assembly during tight junction development.
Kamakura S, Hayase J, Kohda A, Iwakiri Y, Chishiki K, Izaki T, Sumimoto H. Kamakura S, et al. EMBO Rep. 2024 Jan;25(1):144-167. doi: 10.1038/s44319-023-00018-0. Epub 2023 Dec 18. EMBO Rep. 2024. PMID: 38177906 Free PMC article. - The Initial Stage of Tumorigenesis in Drosophila Epithelial Tissues.
Tamori Y. Tamori Y. Adv Exp Med Biol. 2019;1167:87-103. doi: 10.1007/978-3-030-23629-8_5. Adv Exp Med Biol. 2019. PMID: 31520350 Review. - Cdc42 overexpression induces hyperbranching in the developing mammary gland by enhancing cell migration.
Bray K, Gillette M, Young J, Loughran E, Hwang M, Sears JC, Vargo-Gogola T. Bray K, et al. Breast Cancer Res. 2013;15(5):R91. doi: 10.1186/bcr3487. Breast Cancer Res. 2013. PMID: 24074261 Free PMC article. - Oocyte Polarization Is Coupled to the Chromosomal Bouquet, a Conserved Polarized Nuclear Configuration in Meiosis.
Elkouby YM, Jamieson-Lucy A, Mullins MC. Elkouby YM, et al. PLoS Biol. 2016 Jan 7;14(1):e1002335. doi: 10.1371/journal.pbio.1002335. eCollection 2016 Jan. PLoS Biol. 2016. PMID: 26741740 Free PMC article. - Epithelial cell polarity determinant CRB3 in cancer development.
Li P, Mao X, Ren Y, Liu P. Li P, et al. Int J Biol Sci. 2015 Jan 1;11(1):31-7. doi: 10.7150/ijbs.10615. eCollection 2015. Int J Biol Sci. 2015. PMID: 25552927 Free PMC article. Review.
References
- Albrechtsen R, Nielsen M, Wewer U, Engvall E, Ruoslahti E. Basement-membrane changes in breast-cancer detected by immunohistochemical staining for laminin. Cancer Res. 1981;41:5076–81. - PubMed
- An CH, Kim YR, Kim HS, Kim SS, Yoo NJ, Lee SH. Frameshift mutations of vacuolar protein sorting genes in gastric and colorectal cancers with microsatellite instability. Hum Pathol. 2011;43:40–47. - PubMed
- Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, et al. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol. 2006;8:1235–45. - PubMed
- Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources