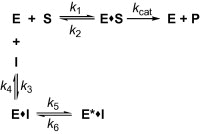Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases - PubMed (original) (raw)
Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases
Ji-Young Park et al. Bioorg Med Chem. 2012.
Abstract
In the search for anti-SARS-CoV, tanshinones derived from Salvia miltiorrhiza were found to be specific and selective inhibitors for the SARS-CoV 3CL(pro) and PL(pro), viral cysteine proteases. A literature search for studies involving the seven isolated tanshinone hits showed that at present, none have been identified as coronaviral protease inhibitors. We have identified that all of the isolated tanshinones are good inhibitors of both cysteine proteases. However, their activity was slightly affected by subtle changes in structure and targeting enzymes. All isolated compounds (1-7) act as time dependent inhibitors of PL(pro), but no improved inhibition was observed following preincubation with the 3CL(pro). In a detail kinetic mechanism study, all of the tanshinones except rosmariquinone (7) were identified as noncompetitive enzyme isomerization inhibitors. However, rosmariquinone (7) showed a different kinetic mechanism through mixed-type simple reversible slow-binding inhibition. Furthermore, tanshinone I (5) exhibited the most potent nanomolar level inhibitory activity toward deubiquitinating (IC(50)=0.7 μM). Additionally, the inhibition is selective because these compounds do not exert significant inhibitory effects against other proteases including chymotrysin, papain, and HIV protease. These findings provide potential inhibitors for SARS-CoV viral infection and replication.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
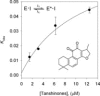
Graphical abstract
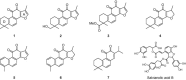
Figure 1
Chemical structures of isolated tanshinones from S. miltiorrhiza lipophilic fraction and representative hydrophilic compound, salvianolic acid B.
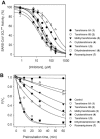
Figure 2
(A) Effects of compounds 1–7 on the activity of SARS-CoV 3CLpro for proteolysis of substrate. (B) SARS-CoV PLpro inhibition as a function of preincubation time for compounds 1–7 at IC50 value.
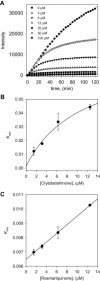
Figure 3
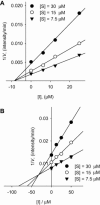
(A) Time course of substrate proteolyzed by PLpro in the presence of compound 4. (B) Dependence of the values for _k_obs on the concentrations of compound 4. (C) Dependence of the values for _k_obs on the concentrations of compound 7. The _k_obs values were fitted to Eqs. (2), (3).
Figure 4
Scheme for time dependent enzyme inhibition. The upper part denoted the turnover of the enzyme in the absence of inhibition. The lower part illustrates the equilibrium for a slow-binding inhibition process. In simple reversible slow-binding inhibition process that the low values of _k_3 and _k_4 relative to enzyme turnover. In enzyme isomerisation, an initial binding of the inhibitor to the enzyme leads to formation of the EI complex, which undergoes an isomerisation of the enzyme to form the new complex E∗I.
Figure 5
Dixon plots for inhibition of compounds 4 and 7 on SARS-CoV PLpro for the proteolysis of substrate. (A) Noncompetitive inhibition of PLpro by compound 4. (B) Mixed-type inhibition of PLpro by compound 7.
Similar articles
- Dieckol, a SARS-CoV 3CL(pro) inhibitor, isolated from the edible brown algae Ecklonia cava.
Park JY, Kim JH, Kwon JM, Kwon HJ, Jeong HJ, Kim YM, Kim D, Lee WS, Ryu YB. Park JY, et al. Bioorg Med Chem. 2013 Jul 1;21(13):3730-7. doi: 10.1016/j.bmc.2013.04.026. Epub 2013 Apr 22. Bioorg Med Chem. 2013. PMID: 23647823 Free PMC article. - Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV.
Park JY, Ko JA, Kim DW, Kim YM, Kwon HJ, Jeong HJ, Kim CY, Park KH, Lee WS, Ryu YB. Park JY, et al. J Enzyme Inhib Med Chem. 2016;31(1):23-30. doi: 10.3109/14756366.2014.1003215. Epub 2015 Feb 16. J Enzyme Inhib Med Chem. 2016. PMID: 25683083 - The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds.
Báez-Santos YM, St John SE, Mesecar AD. Báez-Santos YM, et al. Antiviral Res. 2015 Mar;115:21-38. doi: 10.1016/j.antiviral.2014.12.015. Epub 2014 Dec 29. Antiviral Res. 2015. PMID: 25554382 Free PMC article. Review. - Design, synthesis, and biological evaluation of novel dipeptide-type SARS-CoV 3CL protease inhibitors: structure-activity relationship study.
Thanigaimalai P, Konno S, Yamamoto T, Koiwai Y, Taguchi A, Takayama K, Yakushiji F, Akaji K, Kiso Y, Kawasaki Y, Chen SE, Naser-Tavakolian A, Schön A, Freire E, Hayashi Y. Thanigaimalai P, et al. Eur J Med Chem. 2013 Jul;65:436-47. doi: 10.1016/j.ejmech.2013.05.005. Epub 2013 May 20. Eur J Med Chem. 2013. PMID: 23747811 Free PMC article. - Pharmacological properties of tanshinones, the natural products from Salvia miltiorrhiza.
Wang X, Yang Y, Liu X, Gao X. Wang X, et al. Adv Pharmacol. 2020;87:43-70. doi: 10.1016/bs.apha.2019.10.001. Epub 2020 Jan 13. Adv Pharmacol. 2020. PMID: 32089238 Review.
Cited by
- Dieckol, a SARS-CoV 3CL(pro) inhibitor, isolated from the edible brown algae Ecklonia cava.
Park JY, Kim JH, Kwon JM, Kwon HJ, Jeong HJ, Kim YM, Kim D, Lee WS, Ryu YB. Park JY, et al. Bioorg Med Chem. 2013 Jul 1;21(13):3730-7. doi: 10.1016/j.bmc.2013.04.026. Epub 2013 Apr 22. Bioorg Med Chem. 2013. PMID: 23647823 Free PMC article. - Potential use of polyphenols in the battle against COVID-19.
Paraiso IL, Revel JS, Stevens JF. Paraiso IL, et al. Curr Opin Food Sci. 2020 Apr;32:149-155. doi: 10.1016/j.cofs.2020.08.004. Epub 2020 Sep 9. Curr Opin Food Sci. 2020. PMID: 32923374 Free PMC article. Review. - Natural Products as Potential Leads Against Coronaviruses: Could They be Encouraging Structural Models Against SARS-CoV-2?
Orhan IE, Senol Deniz FS. Orhan IE, et al. Nat Prod Bioprospect. 2020 Aug;10(4):171-186. doi: 10.1007/s13659-020-00250-4. Epub 2020 Jun 11. Nat Prod Bioprospect. 2020. PMID: 32529545 Free PMC article. Review. - An update review of emerging small-molecule therapeutic options for COVID-19.
Tian D, Liu Y, Liang C, Xin L, Xie X, Zhang D, Wan M, Li H, Fu X, Liu H, Cao W. Tian D, et al. Biomed Pharmacother. 2021 May;137:111313. doi: 10.1016/j.biopha.2021.111313. Epub 2021 Feb 3. Biomed Pharmacother. 2021. PMID: 33556871 Free PMC article. Review. - Effect of Co-Solvents, Modified Starch and Physical Parameters on the Solubility and Release Rate of Cryptotanshinone from Alcohologels.
Kobryń J, Demski P, Raszewski B, Zięba T, Musiał W. Kobryń J, et al. Molecules. 2024 Dec 12;29(24):5877. doi: 10.3390/molecules29245877. Molecules. 2024. PMID: 39769966 Free PMC article.
References
- Thiel V., Ivanov K.A., Putics A., Hertzig T., Schelle B., Bayer S., Weissbrich B., Snijder E.J., Rabenau H., Doerr H.W., Grobalenya A.E., Ziebuhr J.J. Gen. Virol. 2003;84:2305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Miscellaneous