Hedgehog controls hepatic stellate cell fate by regulating metabolism - PubMed (original) (raw)
. 2012 Nov;143(5):1319-1329.e11.
doi: 10.1053/j.gastro.2012.07.115. Epub 2012 Aug 8.
Steve S Choi 2, Gregory A Michelotti 1, Isaac S Chan 1, Marzena Swiderska-Syn 1, Gamze F Karaca 1, Guanhua Xie 1, Cynthia A Moylan 2, Francesca Garibaldi 1, Richard Premont 1, Hagir B Suliman 3, Claude A Piantadosi 4, Anna Mae Diehl 5
Affiliations
- PMID: 22885334
- PMCID: PMC3480563
- DOI: 10.1053/j.gastro.2012.07.115
Hedgehog controls hepatic stellate cell fate by regulating metabolism
Yuping Chen et al. Gastroenterology. 2012 Nov.
Abstract
Background & aims: The pathogenesis of cirrhosis, a disabling outcome of defective liver repair, involves deregulated accumulation of myofibroblasts derived from quiescent hepatic stellate cells (HSCs), but the mechanisms that control transdifferentiation of HSCs are poorly understood. We investigated whether the Hedgehog (Hh) pathway controls the fate of HSCs by regulating metabolism.
Methods: Microarray, quantitative polymerase chain reaction, and immunoblot analyses were used to identify metabolic genes that were differentially expressed in quiescent vs myofibroblast HSCs. Glycolysis and lactate production were disrupted in HSCs to determine if metabolism influenced transdifferentiation. Hh signaling and hypoxia-inducible factor 1α (HIF1α) activity were altered to identify factors that alter glycolytic activity. Changes in expression of genes that regulate glycolysis were quantified and localized in biopsy samples from patients with cirrhosis and liver samples from mice following administration of CCl(4) or bile duct ligation. Mice were given systemic inhibitors of Hh to determine if they affect glycolytic activity of the hepatic stroma; Hh signaling was also conditionally disrupted in myofibroblasts to determine the effects of glycolytic activity.
Results: Transdifferentiation of cultured, quiescent HSCs into myofibroblasts induced glycolysis and caused lactate accumulation. Increased expression of genes that regulate glycolysis required Hh signaling and involved induction of HIF1α. Inhibitors of Hh signaling, HIF1α, glycolysis, or lactate accumulation converted myofibroblasts to quiescent HSCs. In diseased livers of animals and patients, numbers of glycolytic stromal cells were associated with the severity of fibrosis. Conditional disruption of Hh signaling in myofibroblasts reduced numbers of glycolytic myofibroblasts and liver fibrosis in mice; similar effects were observed following administration of pharmacologic inhibitors of Hh.
Conclusions: Hedgehog signaling controls the fate of HSCs by regulating metabolism. These findings might be applied to diagnosis and treatment of patients with cirrhosis.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures: The authors disclose no conflicts
Figures
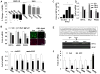
Figure 1. Metabolism is reprogrammed during HSC transdifferentiation
Primary HSCs were cultured for 7 days. Culture-related changes in A) mRNA and B, C) protein expression of glycolytic enzymes, hexokinase (HK2), phosphofructokinase (PFKP), and pyruvate kinase M2 (PKM2). Related changes in B) the MF marker (αSMA), D) glucose transporter (GLUT1), lactate transporter (MCT4), E) gluconeogenesis-related genes, phosphoenolpyruvate carboxykinase (PCK1) and fructose bisphosphatase (FBP1), and F) intracellular lactate. *P<.05; **P<.01; ***P<.001 versus day 0.
Figure 2. Metabolic reprogramming controls the fate of HSCs
Seven-day cultured primary HSCs were analyzed after three-days of treatment with 2-deoxyglucose (2DG, 2.5mM) to inhibit glycolysis (A, B) or FX11 (20μM, LDHA inhibitor) to block lactate generation (C, D). Immunocytochemistry was used to compare effects of 2DG (A) or FX11 (C) on proliferation (BrdU incorporation), expression of MF markers (αSMA), and lipid content (Oil red O). B, D) Changes in gene expression were assessed by QRT-PCR. ***P<.001 versus Day 0; †††P<.001 versus Day 7 control.
Figure 3. Hedgehog signaling controls metabolic reprograming to direct HSC fate
A) QRT-PCR analysis of freshly-isolated HSCs from SMO-LoxP mice that were infected with adenoviral GFP or Cre 2-days before HSC isolation, and freshly-isolated rat primary HSC treated with SAG (Hh agonist, 0.3μM) for 24 hours; B) QRT-PCR analysis and immunostaining of murine HSC treated with GDC-0449 (to inhibit SMO) from culture day 4-7, or culture day 7 SMO-LoxP HSC treated with adenoviral Cre on day 4 (to disrupt the Smo gene); C) QRT-PCR analysis of HIF1α mRNA in primary rat HSC and D) 8B cells transfected with equal amounts of expression constructs for GLI1 or GLI2 (or empty vector) 2 days earlier; E) Chromatin was immunoprecipitated from LX2 cells using GLI2 or IgG antibodies and analyzed by PCR. The GLI consensus sequence within the ChIP amplicon is bolded and underlined. GFAP was used as a specificity control. F), QRT-PCR analysis of culture-day 7 HSCs treated with acriflavine (ACF, HIF1α inhibitor) for three days. *P<.05; **P<.01; ***P<.001 versus control.
Figure 4. Metabolic reprogramming of HSCs is a conserved response to liver injury
Mice injected once with CCl4 (A, B), fed methionine-choline deficient (MCD) diets for 8 weeks (C, D) or subjected to bile duct ligation (BDL, E, F) for 2 weeks to cause liver damage. Changes in glycolysis-related gene/protein expression were evaluated by QRT-PCR (A, C, E), immunoblot and immunohistochemistry (B, D, F) and correlated with changes in MF marker expression (B, D, F and Supplementary Fig.3). *P <.05; **P<.01; ***P<.001 versus control.
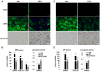
Figure 5. Hedgehog signaling controls metabolic reprogramming during liver injury in vivo
Immunohistochemistry identifies cells expressing the M2 isozyme of PKM2, a specific marker of glycolytic activity, in healthy adult mice (A) and different liver injury models (B-D). Hh signaling was inhibited in PH-mice by cyclopamine (B), in aged MDR2-/- mice by GDC-0449 (C), or abrogated selectively in MF by treating DTG-mice with tamoxifen (D), as indicated.
Figure 6. Hedgehog controls HSC fate by regulating metabolism
Hh ligands released from dying hepatocytes activate Hh signaling in Q-HSC. This inhibits lipogenesis and glucogenogenesis while activating aerobic glycolysis (the Warburg effect). The resultant glycolytic end-products reprogram HSC into proliferative myofibroblasts.
Similar articles
- Hedgehog-YAP Signaling Pathway Regulates Glutaminolysis to Control Activation of Hepatic Stellate Cells.
Du K, Hyun J, Premont RT, Choi SS, Michelotti GA, Swiderska-Syn M, Dalton GD, Thelen E, Rizi BS, Jung Y, Diehl AM. Du K, et al. Gastroenterology. 2018 Apr;154(5):1465-1479.e13. doi: 10.1053/j.gastro.2017.12.022. Epub 2018 Jan 3. Gastroenterology. 2018. PMID: 29305935 Free PMC article. - Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis.
Choi SS, Omenetti A, Witek RP, Moylan CA, Syn WK, Jung Y, Yang L, Sudan DL, Sicklick JK, Michelotti GA, Rojkind M, Diehl AM. Choi SS, et al. Am J Physiol Gastrointest Liver Physiol. 2009 Dec;297(6):G1093-106. doi: 10.1152/ajpgi.00292.2009. Epub 2009 Oct 8. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19815628 Free PMC article. - _Gli2_-regulated activation of hepatic stellate cells and liver fibrosis by TGF-β signaling.
Yan J, Hu B, Shi W, Wang X, Shen J, Chen Y, Huang H, Jin L. Yan J, et al. Am J Physiol Gastrointest Liver Physiol. 2021 May 1;320(5):G720-G728. doi: 10.1152/ajpgi.00310.2020. Epub 2021 Mar 17. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 33728992 - Mitochondria: A critical hub for hepatic stellate cells activation during chronic liver diseases.
Ezhilarasan D. Ezhilarasan D. Hepatobiliary Pancreat Dis Int. 2021 Aug;20(4):315-322. doi: 10.1016/j.hbpd.2021.04.010. Epub 2021 Apr 28. Hepatobiliary Pancreat Dis Int. 2021. PMID: 33975780 Review. - MicroRNAs in liver fibrosis: Focusing on the interaction with hedgehog signaling.
Hyun J, Jung Y. Hyun J, et al. World J Gastroenterol. 2016 Aug 7;22(29):6652-62. doi: 10.3748/wjg.v22.i29.6652. World J Gastroenterol. 2016. PMID: 27547008 Free PMC article. Review.
Cited by
- Curcumin regulates cell fate and metabolism by inhibiting hedgehog signaling in hepatic stellate cells.
Lian N, Jiang Y, Zhang F, Jin H, Lu C, Wu X, Lu Y, Zheng S. Lian N, et al. Lab Invest. 2015 Jul;95(7):790-803. doi: 10.1038/labinvest.2015.59. Epub 2015 May 4. Lab Invest. 2015. PMID: 25938627 - p63 controls metabolic activation of hepatic stellate cells and fibrosis via an HER2-ACC1 pathway.
Fondevila MF, Novoa E, Gonzalez-Rellan MJ, Fernandez U, Heras V, Porteiro B, Parracho T, Dorta V, Riobello C, da Silva Lima N, Seoane S, Garcia-Vence M, Chantada-Vazquez MP, Bravo SB, Senra A, Leiva M, Marcos M, Sabio G, Perez-Fernandez R, Dieguez C, Prevot V, Schwaninger M, Woodhoo A, Martinez-Chantar ML, Schwabe R, Cubero FJ, Varela-Rey M, Crespo J, Iruzubieta P, Nogueiras R. Fondevila MF, et al. Cell Rep Med. 2024 Feb 20;5(2):101401. doi: 10.1016/j.xcrm.2024.101401. Epub 2024 Feb 9. Cell Rep Med. 2024. PMID: 38340725 Free PMC article. - Pyruvate Kinase M2 Tetramerization Protects against Hepatic Stellate Cell Activation and Liver Fibrosis.
Zheng D, Jiang Y, Qu C, Yuan H, Hu K, He L, Chen P, Li J, Tu M, Lin L, Chen H, Lin Z, Lin W, Fan J, Cheng G, Hong J. Zheng D, et al. Am J Pathol. 2020 Nov;190(11):2267-2281. doi: 10.1016/j.ajpath.2020.08.002. Epub 2020 Aug 15. Am J Pathol. 2020. PMID: 32805235 Free PMC article. - Procyanidin B2 inhibits the activation of hepatic stellate cells and angiogenesis via the Hedgehog pathway during liver fibrosis.
Feng J, Wang C, Liu T, Li J, Wu L, Yu Q, Li S, Zhou Y, Zhang J, Chen J, Ji J, Chen K, Mao Y, Wang F, Dai W, Fan X, Wu J, Guo C. Feng J, et al. J Cell Mol Med. 2019 Sep;23(9):6479-6493. doi: 10.1111/jcmm.14543. Epub 2019 Jul 21. J Cell Mol Med. 2019. PMID: 31328391 Free PMC article. - Costunolide reduces glycolysis-associated activation of hepatic stellate cells via inhibition of hexokinase-2.
Ban D, Hua S, Zhang W, Shen C, Miao X, Liu W. Ban D, et al. Cell Mol Biol Lett. 2019 Aug 14;24:52. doi: 10.1186/s11658-019-0179-4. eCollection 2019. Cell Mol Biol Lett. 2019. PMID: 31428167 Free PMC article.
References
- Tsukamoto H. Adipogenic phenotype of hepatic stellate cells. Alcohol Clin Exp Res. 2005;29:132S–133S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL108801/HL/NHLBI NIH HHS/United States
- R37 AA010154/AA/NIAAA NIH HHS/United States
- K08 DK080980/DK/NIDDK NIH HHS/United States
- T32 CA071341/CA/NCI NIH HHS/United States
- R01 DK077794/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases