Recurrent ovarian cancer: is there a role for re-treatment with bevacizumab after an initial complete response to a bevacizumab-containing regimen? - PubMed (original) (raw)
Comparative Study
Recurrent ovarian cancer: is there a role for re-treatment with bevacizumab after an initial complete response to a bevacizumab-containing regimen?
Georgia A McCann et al. Gynecol Oncol. 2012 Nov.
Abstract
Objective: To compare the progression free survival (PFS) and overall survival (OS) in patients with epithelial ovarian cancer (EOC) who received Bev after Bev (BAB) vs. those who were not re-treated with Bev (NOTBev) after initially experiencing a complete response (CR) to a Bev-containing regimen (BCR).
Methods: We performed a retrospective chart review of patients with EOC that received Bev in either the front-line or recurrent setting. Patients who received additional therapy after achieving a CR to BCR were analyzed.
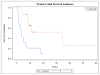
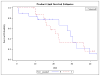
Results: 36 patients who had a CR to a BCR were included, 17 who received Bev at the time of their subsequent recurrence vs. 19 that did not. More patients in the NOTBev group received Bev as primary therapy (21% vs. 6%, p=0.2), but this was not statistically significant. Patients in the BAB group had significantly higher mean PFS compared to the NOTBev group (20 vs. 6 months, p=0.0019). On adjusting for covariates, there was a 78% improvement in their PFS (HR 0.22, p=0.0048). No difference in overall survival was noted between the groups (23 vs. 26 months, p=0.7244).
Conclusions: Re-treatment with Bev after a prior Bev response is associated with a significantly improved PFS. This is the first of such reports in this patient population. The 14-month improvement in PFS strongly supports the re-use of Bev in patients who demonstrate an initial response to Bev. This strategy should be formally tested in future clinical trials and further investigation should include evaluation of predictors of response to Bev therapy.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Figure 1
Progression free survival significantly higher in BAB vs NOTBev group.
Figure 2
No difference in OS between BAB and NOTBev groups.
Figure 3
Clinical response distribution in BAB and NOTBev groups.
Similar articles
- Should bevacizumab be continued after progression on bevacizumab in recurrent ovarian cancer?
Backes FJ, Richardson DL, McCann GA, Smith B, Salani R, Eisenhauer EL, Fowler JM, Copeland LJ, Cohn DE, O'Malley DM. Backes FJ, et al. Int J Gynecol Cancer. 2013 Jun;23(5):833-8. doi: 10.1097/IGC.0b013e318290ea69. Int J Gynecol Cancer. 2013. PMID: 23640292 - Comparison of bevacizumab alone or with chemotherapy in recurrent ovarian cancer patients.
Fuh KC, Secord AA, Bevis KS, Huh W, ElNaggar A, Blansit K, Previs R, Tillmanns T, Kapp DS, Chan JK. Fuh KC, et al. Gynecol Oncol. 2015 Dec;139(3):413-8. doi: 10.1016/j.ygyno.2015.06.041. Epub 2015 Jul 2. Gynecol Oncol. 2015. PMID: 26144600 - Independent radiologic review of the Gynecologic Oncology Group Study 0218, a phase III trial of bevacizumab in the primary treatment of advanced epithelial ovarian, primary peritoneal, or fallopian tube cancer.
Burger RA, Brady MF, Rhee J, Sovak MA, Kong G, Nguyen HP, Bookman MA. Burger RA, et al. Gynecol Oncol. 2013 Oct;131(1):21-6. doi: 10.1016/j.ygyno.2013.07.100. Epub 2013 Jul 29. Gynecol Oncol. 2013. PMID: 23906656 Free PMC article. Clinical Trial. - Treatment of recurrent ovarian cancer.
Pignata S, C Cecere S, Du Bois A, Harter P, Heitz F. Pignata S, et al. Ann Oncol. 2017 Nov 1;28(suppl_8):viii51-viii56. doi: 10.1093/annonc/mdx441. Ann Oncol. 2017. PMID: 29232464 Review. - Angiogenesis inhibitors in the treatment of epithelial ovarian cancer.
Han ES, Wakabayashi M, Leong L. Han ES, et al. Curr Treat Options Oncol. 2013 Mar;14(1):22-33. doi: 10.1007/s11864-012-0220-6. Curr Treat Options Oncol. 2013. PMID: 23288484 Review.
Cited by
- Randomised phase II trial of weekly ixabepilone ± biweekly bevacizumab for platinum-resistant or refractory ovarian/fallopian tube/primary peritoneal cancer.
Roque DM, Siegel ER, Buza N, Bellone S, Silasi DA, Huang GS, Andikyan V, Clark M, Azodi M, Schwartz PE, Rao GG, Reader JC, Hui P, Tymon-Rosario JR, Harold J, Mauricio D, Zeybek B, Menderes G, Altwerger G, Ratner E, Santin AD. Roque DM, et al. Br J Cancer. 2022 Jun;126(12):1695-1703. doi: 10.1038/s41416-022-01717-6. Epub 2022 Feb 11. Br J Cancer. 2022. PMID: 35149854 Free PMC article. Clinical Trial. - Treatment options in recurrent ovarian cancer: latest evidence and clinical potential.
Luvero D, Milani A, Ledermann JA. Luvero D, et al. Ther Adv Med Oncol. 2014 Sep;6(5):229-39. doi: 10.1177/1758834014544121. Ther Adv Med Oncol. 2014. PMID: 25342990 Free PMC article. Review. - Antiangiogenic therapies in ovarian cancer.
Reinthaller A. Reinthaller A. Memo. 2016;9(3):139-143. doi: 10.1007/s12254-016-0282-4. Epub 2016 Sep 15. Memo. 2016. PMID: 27752291 Free PMC article. Review. - Retreatment with bevacizumab in patients with gynecologic malignancy is associated with clinical response and does not increase morbidity.
Laskey RA, Richard SD, Smith AL, Lin JF, Beck TL, Lesnock JL, Kelley JL 3rd, Olawaiye AB, Sukumvanich P, Krivak TC. Laskey RA, et al. Onco Targets Ther. 2014 Mar 21;7:469-76. doi: 10.2147/OTT.S57425. eCollection 2014. Onco Targets Ther. 2014. PMID: 24711703 Free PMC article. - Profile of pazopanib and its potential in the treatment of epithelial ovarian cancer.
Davidson BA, Secord AA. Davidson BA, et al. Int J Womens Health. 2014 Mar 13;6:289-300. doi: 10.2147/IJWH.S49781. eCollection 2014. Int J Womens Health. 2014. PMID: 24648773 Free PMC article. Review.
References
- Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83. - PubMed
- Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–96. - PubMed
- Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25(33):5165–71. - PubMed
- Aghajanian CFN, Rutherford T, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2011;29(18 s, part II of II):781s. - PMC - PubMed
- Guppy AE, Rustin GJ. CA125 response: can it replace the traditional response criteria in ovarian cancer? Oncologist. 2002;7(5):437–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical