Yes-associated protein regulates the hepatic response after bile duct ligation - PubMed (original) (raw)
. 2012 Sep;56(3):1097-107.
doi: 10.1002/hep.25769. Epub 2012 Aug 8.
Nailing Zhang, Yang Xu, Qian Chen, Mehtab Khan, James J Potter, Suresh K Nayar, Toby Cornish, Gianfranco Alpini, Steven Bronk, Duojia Pan, Robert A Anders
Affiliations
- PMID: 22886419
- PMCID: PMC3431197
- DOI: 10.1002/hep.25769
Free PMC article
Yes-associated protein regulates the hepatic response after bile duct ligation
Haibo Bai et al. Hepatology. 2012 Sep.
Free PMC article
Abstract
Human chronic cholestatic liver diseases are characterized by cholangiocyte proliferation, hepatocyte injury, and fibrosis. Yes-associated protein (YAP), the effector of the Hippo tumor-suppressor pathway, has been shown to play a critical role in promoting cholangiocyte and hepatocyte proliferation and survival during embryonic liver development and hepatocellular carcinogenesis. Therefore, the aim of this study was to examine whether YAP participates in the regenerative response after cholestatic injury. First, we examined human liver tissue from patients with chronic cholestasis. We found more-active nuclear YAP in the bile ductular reactions of primary sclerosing cholangitis and primary biliary cirrhosis patient liver samples. Next, we used the murine bile duct ligation (BDL) model to induce cholestatic liver injury. We found significant changes in YAP activity after BDL in wild-type mice. The function of YAP in the hepatic response after BDL was further evaluated with liver-specific Yap conditional deletion in mice. Ablating Yap in the mouse liver not only compromised bile duct proliferation, but also enhanced hepatocyte necrosis and suppressed hepatocyte proliferation after BDL. Furthermore, primary hepatocytes and cholangiocytes isolated from Yap-deficient livers showed reduced proliferation in response to epidermal growth factor in vitro. Finally, we demonstrated that YAP likely mediates its biological effects through the modulation of Survivin expression.
Conclusion: Our data suggest that YAP promotes cholangiocyte and hepatocyte proliferation and prevents parenchymal damage after cholestatic injury in mice and thus may mediate the response to cholestasis-induced human liver disease.
Copyright © 2012 American Association for the Study of Liver Diseases.
Figures
Fig. 1
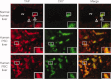
Bile ductular reactions in human PSC and PBC diseased livers show increased nuclear YAP expression and activity. Healthy human liver as well as PSC and PBC patient liver sections were costained with YAP and BEC membranous marker CK7. Upper panels, healthy human liver, showing YAP on the plasma membrane of the portal-tract–associated bile duct (BD) epithelial cells. Middle panel, PSC patient liver. Bottom panel, PBC patient liver. Note uniformly elevated YAP staining throughout the cell, including the nucleus, in BECs of bile ductular reactions. Insets are higher magnifications of representative areas. PV, portal vein, HA, hepatic artery. Original magnification (×20 objective), insets (×40 objective).
Fig. 2
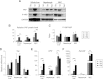
Induction of YAP in the murine liver after experimentally induced cholestasis (A) western blotting analysis. Protein extracts from whole liver, hepatocyte, and BECs of WT mice immediately (0 d) and 5 days (5 d) and 15 days (15 d) after BDL were probed with the indicated antibodies. Note the increase of YAP and phosphorylated YAP (P-YAP) levels in livers 5 days post-BDL. (B and C) YAP protein levels and P-YAP/YAP ratio are quantified in the indicated graphs. Values represent means ± standard error of the mean (SEM) (n = 3-4). *P < 0.05; **P < 0.01; ***P < 0.001; t test. (D-F) Real-time PCR analysis. mRNAs from whole liver, hepatocyte, and BECs of WT mice immediately (0 d), 5 days (5 d), and 15 days (15 d) after BDL were probed with the indicated genes. Note the unchanged Yap mRNA levels in all three components (D) and steadily increasing mRNA levels of BEC markers OPN (E) and EpCAM (F) after BDL in the whole liver. Values represent means ± SEM (n = 3-4). *P < 0.05; **P < 0.01; ***P < 0.001; t test.
Fig. 3
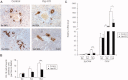
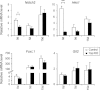
Yap deficiency compromises bile ductular reaction after BDL. (A) Representative liver section stained for the BEC marker, CK19, after BDL. (B) Quantification of CK19-positive cells. Values represent means ± standard error of the mean (SEM) (n = 3-5). ***P < 0.001; t test. (C) Real-time PCR analysis. mRNAs from control and _Yap_-deficient livers immediately (0 d), 5 days (5 d), and 15 days (15 d) after BDL were probed with BEC markers EpCAM and OPN. Note the decreased mRNA levels of EpCAM and OPN in _Yap_-deficient livers, relative to control livers, 15 days post-BDL. Values represent means ± SEM (n = 3-5). *P < 0.05; ***P < 0.001; t test.
Fig. 4
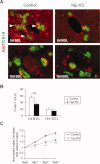
YAP is required for BEC proliferation after BDL. (A) Costaining of proliferation marker Ki67 (red, nucleus) and BEC marker CK19 (green, membrane). White arrowheads point to representative proliferating BECs, which express both CK19 and Ki67. Note the reduced percentage of proliferating BECs in _Yap_-deficient livers, compared to control livers, 5 days post-BDL. (B) Quantification of the percentage of dual positive Ki67+/CK19+ BECs to total BECs (CK19+ only). Values represent means ± standard error of the mean (SEM) (n = 3-5). **P < 0.01; t test. (C) Reduced proliferation response of _Yap_-deficient BECs to EGF stimulation. BECs isolated from control and _Yap_-deficient livers were cultured in vitro in the presence of EGF. Viable cell numbers were measured at indicated time points and plotted as fold of viable cells relative to day 0. Values are means ± SEM (n = 3). **P < 0.01; ***P < 0.001; t test.
Fig. 5
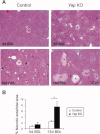
_Yap_-deficient hepatocytes are more susceptible to injury. (A) Representative H&E staining of control and _Yap_-deficient livers 5 and 15 days post-BDL. Regions of hepatocellular necrosis are indicated by asterisks. (B) Quantification of infracted area as a percentage of total area. Values represent means ± standard error of the mean (n = 3-5). *P < 0.05; t test.
Fig. 6
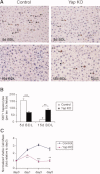
Loss of YAP delays hepatocyte proliferation after BDL. (A) Ki67 staining of control and _Yap_-deficient livers 5 and 15 days post-BDL. Black arrowheads point to Ki67+ hepatocyte nuclei. (B) Quantification of Ki67+ hepatocytes. Values represent means ± standard error of the mean (SEM) (n = 3-5). **P < 0.01; ***P < 0.001; t test. (C) Reduced hepatocyte proliferative response to EGF stimulation. Hepatocytes isolated from control and _Yap_-deficient livers were cultured in vitro in the presence of EGF. Viable cell numbers were measured at indicated time points and plotted as fold of viable cells relative to day 0. Values are means ± SEM (n = 3). *P < 0.05; **P < 0.01; t test.
Fig. 7
mRNA expression of critical BEC mediators in _Yap_-deficient mice after BDL. Real-time PCR analysis for mRNA levels of critical mediators of BEC development and proliferation from control and _Yap_-deficient livers immediately (0 d) or 5 days (5 d) and 15 days (15 d) after BDL. Values represent means ± standard error of the mean (n = 3-5). *P < 0.05; ***P < 0.001; t test.
Fig. 8
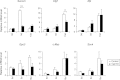
YAP mediates Survivin mRNA expression after BDL. Real-time PCR analysis for mRNA levels genes, which are transcriptionally up-regulated in YAP Tg livers from control and _Yap_-deficient livers immediately (0 d), 5 days (5 d), or 15 days (15 d) after BDL. Note Survivin mRNA levels peak at day 5 post-BDL in WT livers, and this peak was suppressed in _Yap_-deficient livers. Values represent means ± standard error of the mean (n = 3-5). **P < 0.01; ***P < 0.001; t test.
Similar articles
- PGC1α deficiency reverses cholestasis-induced liver injury via attenuating hepatic inflammation and promoting bile duct remodeling.
Li D, Ye C, Liu P, Sun T, Qin Y, Wan X. Li D, et al. Acta Histochem. 2023 Dec;125(8):152097. doi: 10.1016/j.acthis.2023.152097. Epub 2023 Oct 7. Acta Histochem. 2023. PMID: 37813066 - Yes-associated protein regulates the hepatoprotective effect of vitamin D receptor activation through promoting adaptive bile duct remodeling in cholestatic mice.
Xie J, Fan Y, Jia R, Yang F, Ma L, Li L. Xie J, et al. J Pathol. 2021 Sep;255(1):95-106. doi: 10.1002/path.5750. Epub 2021 Jul 16. J Pathol. 2021. PMID: 34156701 - Sortilin Deficiency Reduces Ductular Reaction, Hepatocyte Apoptosis, and Liver Fibrosis in Cholestatic-Induced Liver Injury.
Hubel E, Saroha A, Park WJ, Pewzner-Jung Y, Lavoie EG, Futerman AH, Bruck R, Fishman S, Dranoff JA, Shibolet O, Zvibel I. Hubel E, et al. Am J Pathol. 2017 Jan;187(1):122-133. doi: 10.1016/j.ajpath.2016.09.005. Epub 2016 Nov 11. Am J Pathol. 2017. PMID: 27842214 - Fibrotic Events in the Progression of Cholestatic Liver Disease.
Wu H, Chen C, Ziani S, Nelson LJ, Ávila MA, Nevzorova YA, Cubero FJ. Wu H, et al. Cells. 2021 May 5;10(5):1107. doi: 10.3390/cells10051107. Cells. 2021. PMID: 34062960 Free PMC article. Review. - Role of the Hippo pathway in liver regeneration and repair: recent advances.
Pibiri M, Simbula G. Pibiri M, et al. Inflamm Regen. 2022 Dec 5;42(1):59. doi: 10.1186/s41232-022-00235-5. Inflamm Regen. 2022. PMID: 36471376 Free PMC article. Review.
Cited by
- [Changes of YAP activity at the early stage of nonalcoholic steatohepatitis and its spatiotemporal relationship with ductular reaction in mice].
Liu Y, Liang J, Zeng W, Wang Y. Liu Y, et al. Nan Fang Yi Ke Da Xue Xue Bao. 2022 Sep 20;42(9):1324-1334. doi: 10.12122/j.issn.1673-4254.2022.09.08. Nan Fang Yi Ke Da Xue Xue Bao. 2022. PMID: 36210705 Free PMC article. Chinese. - Biliary NIK promotes ductular reaction and liver injury and fibrosis in mice.
Zhang Z, Zhong X, Shen H, Sheng L, Liangpunsakul S, Lok AS, Omary MB, Wang S, Rui L. Zhang Z, et al. Nat Commun. 2022 Aug 30;13(1):5111. doi: 10.1038/s41467-022-32575-8. Nat Commun. 2022. PMID: 36042192 Free PMC article. - Loss of Mst1/2 activity promotes non-mitotic hair cell generation in the neonatal organ of Corti.
Lu X, Yu H, Ma J, Wang K, Guo L, Zhang Y, Li B, Zhao Z, Li H, Sun S. Lu X, et al. NPJ Regen Med. 2022 Oct 25;7(1):64. doi: 10.1038/s41536-022-00261-4. NPJ Regen Med. 2022. PMID: 36280668 Free PMC article. - Safety Considerations in the Development of Hippo Pathway Inhibitors in Cancers.
Kakiuchi-Kiyota S, Schutten MM, Zhong Y, Crawford JJ, Dey A. Kakiuchi-Kiyota S, et al. Front Cell Dev Biol. 2019 Aug 14;7:156. doi: 10.3389/fcell.2019.00156. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31475147 Free PMC article. Review. - Dynamic YAP expression in the non-parenchymal liver cell compartment controls heterologous cell communication.
Liu K, Wehling L, Wan S, Weiler SME, Tóth M, Ibberson D, Marhenke S, Ali A, Lam M, Guo T, Pinna F, Pedrini F, Damle-Vartak A, Dropmann A, Rose F, Colucci S, Cheng W, Bissinger M, Schmitt J, Birner P, Poth T, Angel P, Dooley S, Muckenthaler MU, Longerich T, Vogel A, Heikenwälder M, Schirmacher P, Breuhahn K. Liu K, et al. Cell Mol Life Sci. 2024 Mar 4;81(1):115. doi: 10.1007/s00018-024-05126-1. Cell Mol Life Sci. 2024. PMID: 38436764 Free PMC article.
References
- Lazaridis KN, Strazzabosco M, LaRusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. - PubMed
- Alvaro D, Mancino MG. New insights on the molecular and cell biology of human cholangiopathies. Mol Aspects Med. 2008;29:50–57. - PubMed
- Schmucker DL, Ohta M, Kanai S, Sato Y, Kitani K. Hepatic injury induced by bile salts: correlation between biochemical and morphological events. HEPATOLOGY. 1990;12:1216–1221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- R01 DK080736/DK/NIDDK NIH HHS/United States
- R01 DK081417/DK/NIDDK NIH HHS/United States
- R01DK081417/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources