Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes - PubMed (original) (raw)
Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes
Justine Bacchetta et al. J Bone Miner Res. 2013 Jan.
Abstract
Vitamin D is a potent stimulator of monocyte innate immunity, and this effect is mediated via intracrine conversion of 25-hydroxyvitamin D (25OHD) to 1,25-dihydroxyvitamin D (1,25(OH)(2) D). In the kidney, synthesis of 1,25(OH)(2) D is suppressed by fibroblast growth factor 23 (FGF23), via transcriptional suppression of the vitamin D-activating enzyme 1α-hydroxylase (CYP27B1). We hypothesized that FGF23 also suppresses CYP27B1 in monocytes, with concomitant effects on intracrine responses to 1,25(OH)(2) D. Healthy donor peripheral blood mononuclear cell monocytes (PBMCm) and peritoneal dialysate monocyte (PDm) effluent from kidney disease patients were assessed at baseline to confirm the presence of mRNA for FGF23 receptors (FGFRs), with Klotho and FGFR1 being more strongly expressed than FGFR2/3/4 in both cell types. Immunohistochemistry showed coexpression of Klotho and FGFR1 in PBMCm and PDm, with this effect being enhanced following treatment with FGF23 in PBMCm but not PDm. Treatment with FGF23 activated mitogen-activated protein kinase (MAPK) and protein kinase B (Akt) pathways in PBMCm, demonstrating functional FGFR signaling in these cells. FGF23 treatment of PBMCm and PDm decreased expression of mRNA for CYP27B1. In PBMCm this was associated with downregulation of 25OHD to 1,25(OH)(2) D metabolism, and concomitant suppression of intracrine induced 24-hydroxylase (CYP24A1) and antibacterial cathelicidin (LL37). FGF23 suppression of CYP27B1 was particularly pronounced in PBMCm treated with interleukin-15 to stimulate synthesis of 1,25(OH)(2) D. These data indicate that FGF23 can inhibit extra-renal expression of CYP27B1 and subsequent intracrine responses to 1,25(OH)(2) D in two different human monocyte models. Elevated expression of FGF23 may therefore play a crucial role in defining immune responses to vitamin D and this, in turn, may be a key determinant of infection in patients with chronic kidney disease (CKD).
Copyright © 2013 American Society for Bone and Mineral Research.
Conflict of interest statement
Disclosure
All authors state that they have no conflicts of interest
Figures
Figure 1. Expression and regulation of fibroblast growth factor receptors (FGFR) and Klotho in PBMCm
1A. Baseline expression of FGFR1 mRNA in PBMCm correlates with Klotho mRNA (Spearman correlation coefficient of 0.585, p<0.001, 35 different batches of PBMCs). 1B. Effect of FGF23 (100 ng/ml) on expression of mRNA Klotho, FGFR1, FGFR2 and FGFR4 in PBMCm in vitro was assessed after 6 hr (black bars) and 24 hr (grey bars) treatments. Data show combined results using PBMCm from healthy donors at 6 hrs (8 donors) and 24 hrs (5 donors). 1C. Effect of FGF23 (100 ng/ml, 24 hrs) on expression of Klotho (red), FGFR-1 (green), and nuclear DAPI (blue) protein in PBMCm, as determined by immunofluoresence microscopy. Merged immunofluoresence shows co-expression of FGFR1 and Klotho in PBMCm.
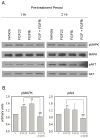
Figure 2. FGF23 modulates FGFR signaling in PBMCm
2A. Effect of FGF23 (100 ng/ml) on MAPK and Aktsignaling in PBMCm. Data are shown as representative Western blots showing expression of protein for: total MAPK; phosphoMAPK (pMAPK); total Akt; phosphoAkt (pAkt). PBMC were pre-treated under conditions of serum deprivation (0.1% human serum) for either 1 hr or 2 hrs with or without an FGFR inhibitor (FGFRi, 250 nM), and then treated with or without vehicle or FGF23 (100 ng/ml) for a further 1 hr. 2B. Quantification of changes in expression of pMAPK (1 hr pre-treatment, left panel) and pAkt (2 hr pre-treatment, right panel) expression normalized to total MAPK and Akt respectively. Data were determined using ImageJ software and represent mean ± SD values for n = 3 separate donor PBMCm cultures. ** = statistically different from vehicle-treated PBMCm, p < 0.01. ## = statistically different from FGF23-treated PBMCm, p < 0.01.
Figure 3. FGF23 suppresses expression and activity of CYP27B1 in PBMCm
3A. Effect of FGF23 (100 ng/ml) at 6hrs (black bars) and 24 hrs (grey bars) on expression of mRNA for CYP27B1, CYP24A1, VDR, LL37 and tumor necrosis factor α (TNFα) in PBMCm. Data shown are mean ± SEM fold-changes in mRNA expression relative to vehicle-treated cells for PBMCm from 8 different healthy donors. 3B. Effect of FGF23 (100 ng/ml, 24 hrs) alone or following pre-activation of cells with interleukin-15 (IL-15, 200 ng/ml, 48 hrs) on CYP27B1 mRNA expression in PBMCm. Data are shown as fold-change in CYP27B1 mRNA relative to vehicle-treated cells for PBMCm from 3 different healthy donors. 3C. Effect of vehicle (V) or FGF23 (100 ng/ml, 24 hrs) alone or following pre-activation of cells with IL-15 (200 ng/ml, 48 hrs) on conversion of 25OHD to 1,25(OH)2D in PBMCm. Data are shown as fmoles 1,25(OH)2D synthesized/hr/106 cells for PBMCm from n = 5 different healthy donors. 3D. Effect of FGF23 (100 ng/ml, 24 hrs) alone or after pre-activation of cells with IL-15 (200 ng/ml, 48 hrs) on conversion of 25OHD to 1,25(OH)2D in PBMCm. Data are shown as representative HLPC analyses for each treatment. * = statistically different compared to vehicle p< 0.05; # = statistically different from IL-15-treated cells, p < 0.05.
Figure 4. FGF23 suppresses expression of CYP27B1 in monocytes from peritoneal dialysates
4A. Effect of FGF23 (100 ng/ml, 6hrs) on expression of mRNA for CYP27B1, CYP24A1, Klotho and FGFR1 in peritoneal dialysate monocytes (PDm). Data are shown as mean ± SEM fold-change in mRNA expression relative to vehicle-treated cells for PDm from n = 7 different donors. 4B. Immunofluorescence analysis of protein for Klotho (green), FGFR-1 (red) and nuclear DAPI (blue) in PDm. Merged immunofluoresence shows co-expression of FGFR1 and Klotho in PDm. * = statistically different from vehicle-treated PDm, p< 0.05.
References
- Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18(6):1637–47. - PubMed
- Yamazaki Y, Tamada T, Kasai N, Urakawa I, Aono Y, Hasegawa H, Fujita T, Kuroki R, Yamashita T, Fukumoto S, Shimada T. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res. 2008;23(9):1509–18. - PubMed
- Yoshiko Y, Wang H, Minamizaki T, Ijuin C, Yamamoto R, Suemune S, Kozai K, Tanne K, Aubin JE, Maeda N. Mineralized tissue cells are a principal source of FGF23. Bone. 2007;40(6):1565–73. - PubMed
- Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2004;19(3):429–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K23 DK080984/DK/NIDDK NIH HHS/United States
- R21 DK091672/DK/NIDDK NIH HHS/United States
- R01 DK035423/DK/NIDDK NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- DK 35423/DK/NIDDK NIH HHS/United States
- P50 AR063020/AR/NIAMS NIH HHS/United States
- UL1 RR033176/RR/NCRR NIH HHS/United States
- DK0911672/DK/NIDDK NIH HHS/United States
- DK 67563/DK/NIDDK NIH HHS/United States
- DK 80984/DK/NIDDK NIH HHS/United States
- R01 DK067563/DK/NIDDK NIH HHS/United States
- P30 AI028697/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous