Cutting edge: a novel mechanism bridging innate and adaptive immunity: IL-12 induction of CD25 to form high-affinity IL-2 receptors on NK cells - PubMed (original) (raw)
Cutting edge: a novel mechanism bridging innate and adaptive immunity: IL-12 induction of CD25 to form high-affinity IL-2 receptors on NK cells
Seung-Hwan Lee et al. J Immunol. 2012.
Abstract
NK cell expression and use of the IL-2Rα-chain (CD25), required for the high-affinity IL-2R, remain poorly understood. The studies reported in this article demonstrate that infections with murine CMV (MCMV), but not with lymphocytic choriomeningitis virus, induce CD25 on NK cells, along with high levels of IL-12 and IL-18. The cytokines act ex vivo to increase CD25 levels, and IL-12, IL-12R, and STAT4, but not the NK activating receptor Ly49H, are required for peak induction in vivo. All examined NK cell populations are driven into proliferation and incorporate BrdU in response to high ex vivo concentrations of IL-2, but only those from MCMV infection respond to low ex vivo concentrations of IL-2. The numbers of NK cells elicited during MCMV infection are reduced by IL-2 neutralization. Thus, a link between innate and adaptive immunity is established by which composition of innate cytokine responses sets up to promote NK cell use of a factor supporting adaptive responses.
Figures
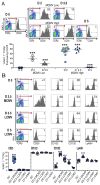
Figure 1. IL-2 receptor expression on NK cells
Mice, B6, were infected with either 5,000 or 50,000 PFU of MCMV, or 4×104 PFU of LCMV. Flow cytometry was used, identifying NK cells within total splenic leukocytes by gating on NK1.1+TCRβ − cells. (A) CD25 expression on D0, D2, D3.5 and D5 of low and high dose MCMV infections are shown. Representative samples from individual mice are given as flow plots. Composites, with data from 6 separate experiments, present symbols for results from individual mice. (B) Evaluated expression of CD25, CD122, CD132, along with the Ly49H activating molecules, is shown. Representative samples prepared from individual mice that were D0, D3.5 high dose MCMV infected, and D3.5 or D5 LCMV infected are given as flow plots. Composites, with data from 3 different experiments including the conditions shown along with studies of D7 LCMV infection, present symbols for results from individual mice. Total numbers of mice examined were from 10-to-13 with the exception of D5 LCMV with 8 and the D7 LCMV with 3. Means ± SE are shown as bars with error spread. P values ≤0.0001 are noted with ***.
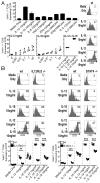
Figure 2. Sensitivity of NK cells to IL-2-induced proliferation
(A) Enriched NK cells from D0 mice and mice infected with high dose MCMV or LCMV on indicated days after infection were stimulated with rIL-2. BrdU incorporation in NK1.1+TCR-β − NK cells was measured. Representative flow cytometry data from stimulation with two doses of rIL-2 (10U/ml and 500U/ml) are shown on left. Summarizes of rIL-2 titrations are on right. These data were compiled from 3 independent experiments for D0, D3.5 MCMV, and D3.5 LCMV; 2 for D5 LCMV; and 1 for D7 LCMV. *The differences between the D3.5 MCMV NK cell responses to all others had P values <0.005. (B) In vivo use of IL-2 was evaluated by blocking IL-2 during infection. The absolute numbers of NK cell (NK1.1+TCRβ −), Ly49H+ NK cells, and Ly49H- yields from spleens of mice either receiving 500 ug of control isotyped-matched Ab or 250 ug each of two α-IL-2 Abs were compared on D1.5 and/or D3.5 after MCMV infection with 5,000 PFU. Each symbol indicates the results from an individual mouse, with 4 per group in each infected group. Means ± SE are shown with error. P values <0.05 are noted with *, and <0.01 with **. Non-significant is denoted as ns.
Figure 3. In vivo requirements for CD25 induction
The expression of CD25 was evaluated, on NK1.1+TCRβ − NK cells, in mice at D0 and D2 of MCMV infection with 5,000 PFU. CD25 expression was compared on NK cells from wt and Ly49H−/− mice (A), from mice either receiving control Ab or α-IL-12/23 Ab at 4h before MCMV infection (B), from wt and IL-12Rβ2−/− mice (C), and from wt and STAT4−/− mice (D). Representative flow plots from individual mice are shown. Composites of results from individual animals are given on the right. Data for panels A, B, and C were assembled from 2 independent experiments with total mice examined on D2 being 6 to 8. The data for panel D are from 1 experiment with 5 to 8 mice per group. Statistically significant differences between groups are indicated (*p<0.01, **p<0.001).
Figure 4. Cytokine induction of CD25 ex vivo
Splenic leukocytes from naïve mice were incubated with indicated cytokines. CD25 expression on NK cells (NK1.1+TCR-β −) was evaluated after 24h stimulation. (A) Responses to a panel of cytokines at the indicated doses. The results are means from 2 separate experiments. Effects of titrated ranges of IL-12, IL-18, or combinations of IL-12 and IL-18 on CD25 induction were determined after 24h stimulation. Each symbol indicates the percentage response from an individual experiment. Experiments were repeated 3 to 4 times. Representative flow cytometric plots from gated NK cells are provided for panel A studies. Mean ± SE of 3 independent experiments for A & B are shown in summaries. CD25 expression on NK cells from wt and IL12Rβ2−/− mice or from wt and STAT4−/− mice (B) after 24h stimulation with individual and combination of IL-12 and IL-18 concentrations are shown. Representative results with gated NK cells are given in histograms. Data are summarized with each symbol representing the value from individual mice. Total numbers of mice for each group were 3. Statistically significant differences between groups are indicated (**p<0.001, ***p<0.0001).
Similar articles
- CD8 T cells in innate immune responses: using STAT4-dependent but antigen-independent pathways to gamma interferon during viral infection.
Suarez-Ramirez JE, Tarrio ML, Kim K, Demers DA, Biron CA. Suarez-Ramirez JE, et al. mBio. 2014 Oct 21;5(5):e01978-14. doi: 10.1128/mBio.01978-14. mBio. 2014. PMID: 25336459 Free PMC article. - Cytokine-Mediated Activation of NK Cells during Viral Infection.
Freeman BE, Raué HP, Hill AB, Slifka MK. Freeman BE, et al. J Virol. 2015 Aug;89(15):7922-31. doi: 10.1128/JVI.00199-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995253 Free PMC article. - Immunoregulatory cytokine networks: 60 years of learning from murine cytomegalovirus.
Biron CA, Tarrio ML. Biron CA, et al. Med Microbiol Immunol. 2015 Jun;204(3):345-54. doi: 10.1007/s00430-015-0412-3. Epub 2015 Apr 8. Med Microbiol Immunol. 2015. PMID: 25850988 Free PMC article. Review. - Innate immunity regulates adaptive immune response: lessons learned from studying the interplay between NK and CD8+ T cells during MCMV infection.
Mitrović M, Arapović J, Traven L, Krmpotić A, Jonjić S. Mitrović M, et al. Med Microbiol Immunol. 2012 Nov;201(4):487-95. doi: 10.1007/s00430-012-0263-0. Epub 2012 Sep 11. Med Microbiol Immunol. 2012. PMID: 22965169 Free PMC article. Review.
Cited by
- Toward the next generation of NK cell-based adoptive cancer immunotherapy.
Ni J, Miller M, Stojanovic A, Cerwenka A. Ni J, et al. Oncoimmunology. 2013 Apr 1;2(4):e23811. doi: 10.4161/onci.23811. Oncoimmunology. 2013. PMID: 23734329 Free PMC article. - Building a Better Defense: Expanding and Improving Natural Killer Cells for Adoptive Cell Therapy.
Maia A, Tarannum M, Lérias JR, Piccinelli S, Borrego LM, Maeurer M, Romee R, Castillo-Martin M. Maia A, et al. Cells. 2024 Mar 5;13(5):451. doi: 10.3390/cells13050451. Cells. 2024. PMID: 38474415 Free PMC article. Review. - What Fuels Natural Killers? Metabolism and NK Cell Responses.
Gardiner CM, Finlay DK. Gardiner CM, et al. Front Immunol. 2017 Apr 3;8:367. doi: 10.3389/fimmu.2017.00367. eCollection 2017. Front Immunol. 2017. PMID: 28421073 Free PMC article. Review. - The Multifaceted Roles of NK Cells in the Context of Murine Cytomegalovirus and Lymphocytic Choriomeningitis Virus Infections.
Hamdan TA. Hamdan TA. Immune Netw. 2024 Jun 27;24(4):e29. doi: 10.4110/in.2024.24.e29. eCollection 2024 Aug. Immune Netw. 2024. PMID: 39246620 Free PMC article. Review. - Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells.
Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA, Fehniger TA. Leong JW, et al. Biol Blood Marrow Transplant. 2014 Apr;20(4):463-73. doi: 10.1016/j.bbmt.2014.01.006. Epub 2014 Jan 13. Biol Blood Marrow Transplant. 2014. PMID: 24434782 Free PMC article.
References
- French AR, Sjolin H, Kim S, Koka R, Yang L, Young DA, Cerboni C, Tomasello E, Ma A, Vivier E, Karre K, Yokoyama WM. DAP12 signaling directly augments proproliferative cytokine stimulation of NK cells during viral infections. J Immunol. 2006;177:4981–4990. - PubMed
- Fu X, Liu Y, Li L, Li Q, Qiao D, Wang H, Lao S, Fan Y, Wu C. Human natural killer cells expressing the memory-associated marker CD45RO from tuberculous pleurisy respond more strongly and rapidly than CD45RO-natural killer cells following stimulation with interleukin-12. Immunology. 2011;134:41–49. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous