Contributions of the striatum to learning, motivation, and performance: an associative account - PubMed (original) (raw)
Review
Contributions of the striatum to learning, motivation, and performance: an associative account
Mimi Liljeholm et al. Trends Cogn Sci. 2012 Sep.
Abstract
It has long been recognized that the striatum is composed of distinct functional sub-units that are part of multiple cortico-striatal-thalamic circuits. Contemporary research has focused on the contribution of striatal sub-regions to three main phenomena: learning of associations between stimuli, actions and rewards; selection between competing response alternatives; and motivational modulation of motor behavior. Recent proposals have argued for a functional division of the striatum along these lines, attributing, for example, learning to one region and performance to another. Here, we consider empirical data from human and animal studies, as well as theoretical notions from both the psychological and computational literatures, and conclude that striatal sub-regions instead differ most clearly in terms of the associations being encoded in each region.
Copyright © 2012. Published by Elsevier Ltd.
Figures
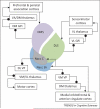
Figure 1
Schematic representation of corticostriatal connections based on [1,2,87,88]. Different cortical areas project to different sub-regions of the striatum, which then project back to respective cortical areas via the internal segment of the globus pallidus (GPi) and the thalamus (direct pathway). Not shown projections include those from different striatal sub-regions to distinct areas in the substantia nigra pars reticulata (SNr) and the external segment of the globus pallidus, as well as those from distinct midbrain nuclei (e.g., substantia nigra compacta and ventral tegmental area) to different striatal sub-regions. Moreover, in the ventral striatum, the Nacc shell, but not the core, projects heavily to the amygdala and lateral hypothalamus, whereas both the shell and core receive inputs from limbic regions (e.g., amygdala and hippocampus). Circle, inhibitory connection; Arrow, excitatory connection; DMS, dorsomedial striatum; DLS, dorsolateral striatum; GPi, internal segment of globus pallidus; VP, ventral pallidum; VA, ventral anterior; DM, dorsomedial; VL, ventrolateral; VM, ventromedial; Nacc C, nucleus accumbens core; Nacc Sh, nucleus accumbens shell.
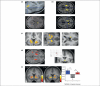
Figure 2
Human neuroimaging studies that indicate differential contributions of striatal sub-regions in associative encoding. (a) Striatal activity tracking the development of habits in posterior lateral striatum (top) and during goal-directed instrumental performance in anterior DMS (bottom). Reproduced, with permission, from [23] and [22], respectively. (b) Activity in the lateral striatum (top) as participants executed a well-trained motor sequence and in the DMS (bottom) as participants planned performance of a self-generated, novel, motor sequence. Reproduced, with permission, from [5]. No effects were found in the DMS when participants planned performance of a well-trained sequence (condition not shown here). (c) Effects in the VS (left) for the conjunction of high versus low incentives and correlation with performance levels. Activity in the lateral striatum is correlated with VS activity when the task entails high demands on motor performance (center), while activity in the medial striatum (right) correlates with the VS signal during high demands on cognitive performance. Reproduced, with permission, from [13]. (d) Imaging effects in the lateral VS for specific PIT (left) and in the medial VS for general PIT (right). Reproduced, with permission, from [47] and [46] respectively. (e) BOLD responses in the VS in anticipation of monetary gain (left) and loss (right), with % signal change shown in bar graph on far right, for gain (blue), no outcome (gray), and loss (red). Reproduced, with permission, from [64].
Similar articles
- The rewarding value of good motor performance in the context of monetary incentives.
Lutz K, Pedroni A, Nadig K, Luechinger R, Jäncke L. Lutz K, et al. Neuropsychologia. 2012 Jul;50(8):1739-47. doi: 10.1016/j.neuropsychologia.2012.03.030. Epub 2012 May 6. Neuropsychologia. 2012. PMID: 22569215 - Modulation of associative learning in the hippocampal-striatal circuit based on item-set similarity.
Stark SM, Frithsen A, Mattfeld AT, Stark CEL. Stark SM, et al. Cortex. 2018 Dec;109:60-73. doi: 10.1016/j.cortex.2018.08.035. Epub 2018 Sep 18. Cortex. 2018. PMID: 30300757 Free PMC article. - Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior.
Hollerman JR, Tremblay L, Schultz W. Hollerman JR, et al. Prog Brain Res. 2000;126:193-215. doi: 10.1016/S0079-6123(00)26015-9. Prog Brain Res. 2000. PMID: 11105648 Review. - Elderly adults show higher ventral striatal activation in response to motor performance related rewards than young adults.
Widmer M, Stulz S, Luft AR, Lutz K. Widmer M, et al. Neurosci Lett. 2017 Nov 20;661:18-22. doi: 10.1016/j.neulet.2017.09.038. Epub 2017 Sep 20. Neurosci Lett. 2017. PMID: 28939388 - Striatal contributions to reward and decision making: making sense of regional variations in a reiterated processing matrix.
Wickens JR, Budd CS, Hyland BI, Arbuthnott GW. Wickens JR, et al. Ann N Y Acad Sci. 2007 May;1104:192-212. doi: 10.1196/annals.1390.016. Epub 2007 Apr 7. Ann N Y Acad Sci. 2007. PMID: 17416920 Review.
Cited by
- Cortical and subcortical contributions to sequence retrieval: Schematic coding of temporal context in the neocortical recollection network.
Hsieh LT, Ranganath C. Hsieh LT, et al. Neuroimage. 2015 Nov 1;121:78-90. doi: 10.1016/j.neuroimage.2015.07.040. Epub 2015 Jul 22. Neuroimage. 2015. PMID: 26209802 Free PMC article. - Remembrance of happy things past: positive autobiographical memories are intrinsically rewarding and valuable, but not in depression.
Chen C, Takahashi T, Yang S. Chen C, et al. Front Psychol. 2015 Mar 3;6:222. doi: 10.3389/fpsyg.2015.00222. eCollection 2015. Front Psychol. 2015. PMID: 25784888 Free PMC article. No abstract available. - GPR88 in D1R-Type and D2R-Type Medium Spiny Neurons Differentially Regulates Affective and Motor Behavior.
Meirsman AC, Ben Hamida S, Clarke E, de Kerchove d'Exaerde A, Darcq E, Kieffer BL. Meirsman AC, et al. eNeuro. 2019 Aug 8;6(4):ENEURO.0035-19.2019. doi: 10.1523/ENEURO.0035-19.2019. Print 2019 Jul/Aug. eNeuro. 2019. PMID: 31346000 Free PMC article. - Dopamine neuron glutamate cotransmission evokes a delayed excitation in lateral dorsal striatal cholinergic interneurons.
Chuhma N, Mingote S, Yetnikoff L, Kalmbach A, Ma T, Ztaou S, Sienna AC, Tepler S, Poulin JF, Ansorge M, Awatramani R, Kang UJ, Rayport S. Chuhma N, et al. Elife. 2018 Oct 8;7:e39786. doi: 10.7554/eLife.39786. Elife. 2018. PMID: 30295607 Free PMC article. - Striatal circuits for reward learning and decision-making.
Cox J, Witten IB. Cox J, et al. Nat Rev Neurosci. 2019 Aug;20(8):482-494. doi: 10.1038/s41583-019-0189-2. Nat Rev Neurosci. 2019. PMID: 31171839 Free PMC article. Review.
References
- Lynd-Balta E, Haber SN. The organization of midbrain projections to the striatum in the primate: sensorimotor-related striatum versus ventral striatum. Neuroscience. 1994;59:625–640. - PubMed
- Zahm DS, et al. Ventral striatopallidothalamic projection: IV. Relative involvements of neurochemically distinct subterritories in the ventral pallidum and adjacent parts of the rostroventral forebrain. J. Comp. Neurol. 1996;364:340–362. - PubMed
- Balleine B, et al. Multiple forms of value learning and the function of dopamine. In: Glimcher PW, et al., editors. Neuroeconomics: Decision Making and the Brain. Academic Press; 2008.
- Balleine BW, et al. The integrative function of the basal ganglia in instrumental conditioning. Behav. Brain Res. 2009;199:43–52. - PubMed
- Jankowski J, et al. Distinct striatal regions for planning and executing novel and automated movement sequences. Neuroimage. 2009;44:1369–1379. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources