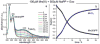Multicopper oxidase involvement in both Mn(II) and Mn(III) oxidation during bacterial formation of MnO(2) - PubMed (original) (raw)
Multicopper oxidase involvement in both Mn(II) and Mn(III) oxidation during bacterial formation of MnO(2)
Alexandra V Soldatova et al. J Biol Inorg Chem. 2012 Dec.
Abstract
Global cycling of environmental manganese requires catalysis by bacteria and fungi for MnO(2) formation, since abiotic Mn(II) oxidation is slow under ambient conditions. Genetic evidence from several bacteria indicates that multicopper oxidases (MCOs) are required for MnO(2) formation. However, MCOs catalyze one-electron oxidations, whereas the conversion of Mn(II) to MnO(2) is a two-electron process. Trapping experiments with pyrophosphate (PP), a Mn(III) chelator, have demonstrated that Mn(III) is an intermediate in Mn(II) oxidation when mediated by exosporium from the Mn-oxidizing bacterium Bacillus SG-1. The reaction of Mn(II) depends on O(2) and is inhibited by azide, consistent with MCO catalysis. We show that the subsequent conversion of Mn(III) to MnO(2) also depends on O(2) and is inhibited by azide. Thus, both oxidation steps appear to be MCO-mediated, likely by the same enzyme, which is indicated by genetic evidence to be the MnxG gene product. We propose a model of how the manganese oxidase active site may be organized to couple successive electron transfers to the formation of polynuclear Mn(IV) complexes as precursors to MnO(2) formation.
Figures
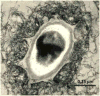
Fig. 1. TEM of MnO2-coated spores from Bacillus sp. SG-1 [9]
Fig. 2
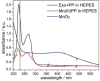
UV-vis absorption spectra of exosporium (50 μL in 1 mL of HEPES buffer) (black), Mn(III)-pyrophosphate complex (100 μM in HEPES buffer) (red), and MnO2 suspension in water (blue) used as basis spectra for a linear squares fit analysis of the UV-vis absorption spectra taken over 30 h experiments of Mn oxidation catalyzed by exosporium
Fig. 3
Time-resolved UV-vis absorption spectral measurements of Mn(II) oxidation by exosporium at room temperature. (a) Selected UV-vis absorption spectra of the reaction mixture taken at the indicated time points during the course of Mn(II) oxidation. The growth of 265 nm band indicates formation of the Mn(III) intermediate trapped by pyrophosphate; the 400 nm band is due to formation of particulate Mn oxides. (b) Time courses followed by Mn(III)-pyrophosphate and MnO2 species during the 30 h UV-vis absorption experiment and obtained from the fit of the absorption spectrum at each time point to a linear combination of the component spectra depicted in Fig.2
Fig. 4
Time-resolved UV-vis absorption spectral measurements of Mn(III) oxidation by exosporium at room temperature. (a) Selected UV-vis absorption spectra of the reaction mixture taken at the indicated time points during Mn(III) oxidation. The growth of the 400 nm band indicates formation of the particulate Mn oxides. (b) Time courses followed by Mn(III)-pyrophosphate and MnO2 species during the 30 h UV-vis absorption experiment and obtained from the fit of the absorption spectrum at each time point to a linear combination of the component spectra depicted in Fig. 2
Fig. 5
UV-vis absorption spectra of the reaction mixture taken at the indicated time points during Mn(III) oxidation by exosporium (a) under anaerobic condition; (b) with 10 mM azide present. Both conditions, exclusion of oxygen and addition of azide, resulted in inhibition of Mn(III) oxidation
Fig. 6
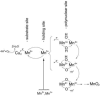
Possible pathways for bacterial MnO2 formation by MCO-catalyzed Mn(II) to Mn(III) oxidation followed by further oxidation (top) or by disproportionation (bottom) of complexed Mn(III)
Fig. 7
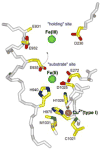
Structure of human ceruloplasmin (pdb #:1KCW) showing mononuclear type 1 copper site, and “substrate” and “holding” cation-binding sites in domain 6. Arrow indicates a movement of sidechain E935 from the substrate site to the holding site upon iron translocation after oxidation. Adapted from ref. [28]
Fig. 8. Proposed mechanism of Mn(II) oxidation and MnO2 formation catalyzed by manganese oxidase, MnxG (see text for details)
Similar articles
- Bacteriogenic manganese oxides.
Spiro TG, Bargar JR, Sposito G, Tebo BM. Spiro TG, et al. Acc Chem Res. 2010 Jan 19;43(1):2-9. doi: 10.1021/ar800232a. Acc Chem Res. 2010. PMID: 19778036 Review. - Mn(II,III) oxidation and MnO2 mineralization by an expressed bacterial multicopper oxidase.
Butterfield CN, Soldatova AV, Lee SW, Spiro TG, Tebo BM. Butterfield CN, et al. Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):11731-5. doi: 10.1073/pnas.1303677110. Epub 2013 Jul 1. Proc Natl Acad Sci U S A. 2013. PMID: 23818588 Free PMC article. - Mn(II) Oxidation by the Multicopper Oxidase Complex Mnx: A Coordinated Two-Stage Mn(II)/(III) and Mn(III)/(IV) Mechanism.
Soldatova AV, Romano CA, Tao L, Stich TA, Casey WH, Britt RD, Tebo BM, Spiro TG. Soldatova AV, et al. J Am Chem Soc. 2017 Aug 23;139(33):11381-11391. doi: 10.1021/jacs.7b02772. Epub 2017 Aug 15. J Am Chem Soc. 2017. PMID: 28712303 - Mn(II) Oxidation by the Multicopper Oxidase Complex Mnx: A Binuclear Activation Mechanism.
Soldatova AV, Tao L, Romano CA, Stich TA, Casey WH, Britt RD, Tebo BM, Spiro TG. Soldatova AV, et al. J Am Chem Soc. 2017 Aug 23;139(33):11369-11380. doi: 10.1021/jacs.7b02771. Epub 2017 Aug 15. J Am Chem Soc. 2017. PMID: 28712284 - The molecular biogeochemistry of manganese(II) oxidation.
Geszvain K, Butterfield C, Davis RE, Madison AS, Lee SW, Parker DL, Soldatova A, Spiro TG, Luther GW, Tebo BM. Geszvain K, et al. Biochem Soc Trans. 2012 Dec 1;40(6):1244-8. doi: 10.1042/BST20120229. Biochem Soc Trans. 2012. PMID: 23176462 Review.
Cited by
- Production of Manganese Oxide Nanoparticles by Shewanella Species.
Wright MH, Farooqui SM, White AR, Greene AC. Wright MH, et al. Appl Environ Microbiol. 2016 Aug 15;82(17):5402-9. doi: 10.1128/AEM.00663-16. Print 2016 Sep 1. Appl Environ Microbiol. 2016. PMID: 27342559 Free PMC article. - Mn(III) species formed by the multi-copper oxidase MnxG investigated by electron paramagnetic resonance spectroscopy.
Tao L, Stich TA, Soldatova AV, Tebo BM, Spiro TG, Casey WH, Britt RD. Tao L, et al. J Biol Inorg Chem. 2018 Oct;23(7):1093-1104. doi: 10.1007/s00775-018-1587-z. Epub 2018 Jul 2. J Biol Inorg Chem. 2018. PMID: 29968177 - Mn oxide formation by phototrophs: Spatial and temporal patterns, with evidence of an enzymatic superoxide-mediated pathway.
Chaput DL, Fowler AJ, Seo O, Duhn K, Hansel CM, Santelli CM. Chaput DL, et al. Sci Rep. 2019 Dec 3;9(1):18244. doi: 10.1038/s41598-019-54403-8. Sci Rep. 2019. PMID: 31796791 Free PMC article. - Biogenic manganese oxide nanoparticle formation by a multimeric multicopper oxidase Mnx.
Romano CA, Zhou M, Song Y, Wysocki VH, Dohnalkova AC, Kovarik L, Paša-Tolić L, Tebo BM. Romano CA, et al. Nat Commun. 2017 Sep 29;8(1):746. doi: 10.1038/s41467-017-00896-8. Nat Commun. 2017. PMID: 28963463 Free PMC article. - Microbial magnetite oxidation via MtoAB porin-multiheme cytochrome complex in Sideroxydans lithotrophicus ES-1.
Keffer JL, Zhou N, Rushworth DD, Yu Y, Chan CS. Keffer JL, et al. bioRxiv [Preprint]. 2025 Jan 23:2024.09.20.614158. doi: 10.1101/2024.09.20.614158. bioRxiv. 2025. PMID: 39345469 Free PMC article. Updated. Preprint.
References
- Nelson YM, Lion LW. In: Geochemical and Hydrological Reactivity of Heavy Metals in Soils. Selim HM, Kingerly WL, editors. CRC Press; Boca Raton: 2003.
- Tebo BM, Bargar JR, Clement BG, Dick GJ, Murray KJ, Parker D, Verity R, Webb SM. Annu Rev Earth Planet Sci. 2004;32:287–328.
- Morgan JJ. Metal Ions in Biological Systems. Marcel Dekker; New York: 2000.
- Morgan JJ. Geochim Cosmochim Acta. 2005;69:35–48.
- Spiro TG, Bargar JR, Sposito G, Tebo BM. Acc Chem Res. 2010;43:2–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous