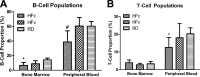Bone structure and B-cell populations, crippled by obesity, are partially rescued by brief daily exposure to low-magnitude mechanical signals - PubMed (original) (raw)
Bone structure and B-cell populations, crippled by obesity, are partially rescued by brief daily exposure to low-magnitude mechanical signals
M Ete Chan et al. FASEB J. 2012 Dec.
Abstract
Deterioration of the immune and skeletal systems, each of which parallel obesity, reflects a fragile interrelationship between adiposity and osteoimmunology. Using a murine model of diet-induced obesity, this study investigated the ability of mechanical signals to protect the skeletal-immune systems at the tissue, cellular, and molecular level. A long-term (7 mo) high-fat diet increased total adiposity (+62%), accelerated age-related loss of trabecular bone (-61%), and markedly reduced B-cell number in the marrow (-52%) and blood (-36%) compared to mice fed a regular diet. In the final 4 mo of the protocol, the application of low-magnitude mechanical signals (0.2 g at 90 Hz, 15 min/d, 5 d/wk) restored both bone structure and B cells to those levels measured in control mice fed a regular diet. These phenotypic outcomes were achieved, in part, by reductions in osteoclastic activity and a biasing of hematopoietic stem cell differentiation toward the lymphoid B-cell lineage and away from a myeloid fate. These results emphasize that obesity undermines both the skeletal and immune systems, yet brief exposure to mechanical signals, perhaps as a surrogate to the salutary influence of exercise, diminishes the consequences of diabetes and obesity, restoring bone structure and normalizing B-cell populations by biasing of the fate of stem cells through mechanosensitive pathways.
Figures
Figure 1.
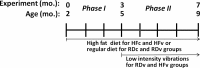
Timeline showing two phases of the experiment. Phase I, which began when the mice were 2 mo of age, involved only differences in diet: according to their assigned groups, mice were fed either a high-fat diet (HF) or a regular diet (RD) for 3 mo, allowing the obese phenotype to be fully established by 5 mo of age. Phase II then tested the effects of combined challenges of diet plus LIV in RDv and HFv groups, while RDc and HFc groups remained on the same diet treatment and were sham-loaded. While no differences were measured between RDc and RDv groups by the end of phase II, the obese phenotype created in the HFc group markedly accelerated bone loss between 5 and 9 mo of age, and suppressed the immune system, as indicated by compromised B-cell activity. HFv treatment, while slightly reducing adiposity relative to HFc treatment, served to restore the bone and reestablish B-cell levels to those in RD groups.
Figure 2.
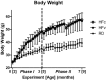
Change of body mass over two phases of the experiment. Phase I spanned 0 to 3 mo of the protocol, which brought mice from 2 to 5 mo of age. Phase II, beginning at dashed line, spanned 3 to 7 mo of the protocol, which brought mice from 5 to 9 mo of age. While marked increases in body mass between the HF and RD groups were found through the entire protocol, the mechanical intervention (RDv and HFv treatment) did not cause a divergence in mass from the control groups. Body mass of HF groups are significantly higher than RD groups starting at the fourth week, and extended through the remainder of the study.
Figure 3.
A) Transverse in vivo μCT sections of the abdominal region of an HFc mouse (top) and an RDc mouse (bottom) at the end of the 7-mo protocol. Images show the adipose tissue in the subcutaneous (gray) and visceral (pink) regions, indicating the increased visceral fat burden caused by HF treatment. B) Abdominal TAT volumes quantified at the end of phases I and II indicate a continued increase in all groups, but a slowed increase in the HFv group. C, D) TAT volumes were subdivided into visceral (C) and subcutaneous (D) regions for individual analysis, and emphasize the major differences in adipose tissue that were apparent in the visceral cavity. *P < 0.05 vs. pooled RD groups.
Figure 4.
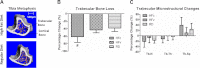
A) Reconstructed μCT images of the tibial metaphysis of a HFc (top) and RDc (bottom) mouse, showing the trabecular (pink) and cortical bone (gray) at the end of the 7 mo protocol. B) All mice experienced age-related trabecular bone loss from the end of phase I through the end of phase II, spanning 5 to 9 mo of age. However, HF treatment accelerated that bone loss in the HFc group, while the mechanical signals in HFv treatment restored values to those measured in the RD mice. C) Accelerated loss of bone in the HFc group was an aggregate of nonsignificant decreases in trabecular number (Tb.N) and thickness (Tb.Th), paralleled by increases in trabecular spacing (Tb.Sp). #P < 0.05 vs. HFv and pooled RD groups.
Figure 5.
A) Flow cytometry analysis of the B-cell populations in bone marrow and peripheral blood, showing the detrimental effect of a long-term high-fat diet in HFc mice, as indicated by significant reduction in cell proportions relative to RD mice. The mechanical signal in HFv treatment restored the B-cell values such that they were not significantly different from RD groups. B) T-cell population of HFc mice exhibited significant reduction in the peripheral blood but not in the bone marrow, while HFv mice showed a trend toward recovery. *P < 0.05 vs. RD groups; #P < 0.05 vs. HFv and pooled RD groups.
Figure 6.
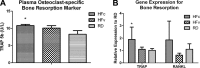
Acceleration and mitigation of bone loss by HF and LIV treatment, respectively, were further investigated at the molecular and transcriptional levels. Analysis of the plasma level of osteoclast-specific bone resorption biomarker TRAP-5b using ELISA (A) and expression levels of bone-resorption genes TRAP and RANKL using real-time rt-PCR (B) commonly showed up-regulation in the HFc compared to RD groups, indicating a contributing mechanism toward the accelerated loss of trabecular bone. Mechanical stimulation in the HFv group attenuated gene expression and bone-resorption biomarker for bone resorption, which may in part explain the rescue of trabecular bone relative to the RD mice. *P < 0.05 vs. pooled RD groups.
Figure 7.
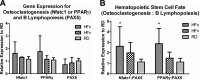
Osteoclasts and B lymphocytes share the same HSC progenitors in the bone marrow niche. A) Elevated expression of Nfatc1 and PPARγ indicated increased osteoclastogenesis, while lower PAX5 expression indicated reduced B lymphopoiesis in HFc relative to RD groups. B) Relative ratio of gene expression involved in osteoclastogenesis to B lymphopoiesis indicated how a long-term high-fat diet significantly biased HSC differentiation toward an osteoclast lineage and away from B-cell lineage in HFc compared to RD groups, while LIV ameliorated such obesity-induced bias of HSC differentiation in HFv group. *P < 0.05 vs. pooled RD groups.
Similar articles
- Ionizing Radiation Stimulates Expression of Pro-Osteoclastogenic Genes in Marrow and Skeletal Tissue.
Alwood JS, Shahnazari M, Chicana B, Schreurs AS, Kumar A, Bartolini A, Shirazi-Fard Y, Globus RK. Alwood JS, et al. J Interferon Cytokine Res. 2015 Jun;35(6):480-7. doi: 10.1089/jir.2014.0152. Epub 2015 Mar 3. J Interferon Cytokine Res. 2015. PMID: 25734366 Free PMC article. - Voluntary running of defined distances alters bone microstructure in C57BL/6 mice fed a high-fat diet.
Yan L, Nielsen FH, Sundaram S, Cao J. Yan L, et al. Appl Physiol Nutr Metab. 2021 Nov;46(11):1337-1344. doi: 10.1139/apnm-2021-0061. Epub 2021 May 17. Appl Physiol Nutr Metab. 2021. PMID: 34000207 - Tissue-specific expression of Sprouty1 in mice protects against high-fat diet-induced fat accumulation, bone loss and metabolic dysfunction.
Urs S, Henderson T, Le P, Rosen CJ, Liaw L. Urs S, et al. Br J Nutr. 2012 Sep 28;108(6):1025-33. doi: 10.1017/S0007114511006209. Epub 2011 Dec 6. Br J Nutr. 2012. PMID: 22142492 Free PMC article. - Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity.
Pagnotti GM, Styner M, Uzer G, Patel VS, Wright LE, Ness KK, Guise TA, Rubin J, Rubin CT. Pagnotti GM, et al. Nat Rev Endocrinol. 2019 Jun;15(6):339-355. doi: 10.1038/s41574-019-0170-1. Nat Rev Endocrinol. 2019. PMID: 30814687 Free PMC article. Review. - Bone metabolism in obesity and weight loss.
Shapses SA, Sukumar D. Shapses SA, et al. Annu Rev Nutr. 2012 Aug 21;32:287-309. doi: 10.1146/annurev.nutr.012809.104655. Annu Rev Nutr. 2012. PMID: 22809104 Free PMC article. Review.
Cited by
- Obesity, Bone Loss, and Periodontitis: The Interlink.
Zhao P, Xu A, Leung WK. Zhao P, et al. Biomolecules. 2022 Jun 22;12(7):865. doi: 10.3390/biom12070865. Biomolecules. 2022. PMID: 35883424 Free PMC article. Review. - Physical energies to the rescue of damaged tissues.
Facchin F, Canaider S, Tassinari R, Zannini C, Bianconi E, Taglioli V, Olivi E, Cavallini C, Tausel M, Ventura C. Facchin F, et al. World J Stem Cells. 2019 Jun 26;11(6):297-321. doi: 10.4252/wjsc.v11.i6.297. World J Stem Cells. 2019. PMID: 31293714 Free PMC article. Review. - Immunometabolism at the Heart of Cardiovascular Disease.
DeBerge M, Chaudhary R, Schroth S, Thorp EB. DeBerge M, et al. JACC Basic Transl Sci. 2023 Apr 26;8(7):884-904. doi: 10.1016/j.jacbts.2022.12.010. eCollection 2023 Jul. JACC Basic Transl Sci. 2023. PMID: 37547069 Free PMC article. Review. - Dysregulation of Leukocyte Trafficking in Type 2 Diabetes: Mechanisms and Potential Therapeutic Avenues.
Pezhman L, Tahrani A, Chimen M. Pezhman L, et al. Front Cell Dev Biol. 2021 Feb 22;9:624184. doi: 10.3389/fcell.2021.624184. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33692997 Free PMC article. Review. - The skeleton in a physical world.
Rubin J, Styner M. Rubin J, et al. Exp Biol Med (Maywood). 2022 Dec;247(24):2213-2222. doi: 10.1177/15353702221113861. Epub 2022 Aug 19. Exp Biol Med (Maywood). 2022. PMID: 35983849 Free PMC article. Review.
References
- Kahn S. E., Zinman B., Haffner S. M., O'Neill M. C., Kravitz B. G., Yu D., Freed M. I., Herman W. H., Holman R. R., Jones N. P., Lachin J. M., Viberti G. C. (2006) Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes 55, 2357–2364 - PubMed
- Cao J. J., Sun L., Gao H. (2010) Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Ann. N. Y. Acad. Sci. 1192, 292–297 - PubMed
- Compston J. E., Watts N. B., Chapurlat R., Cooper C., Boonen S., Greenspan S., Pfeilschifter J., Silverman S., Diez-Perez A., Lindsay R., Saag K. G., Netelenbos J. C., Gehlbach S., Hooven F. H., Flahive J., Adachi J. D., Rossini M., Lacroix A. Z., Roux C., Sambrook P. N., Siris E. S. (2011) Obesity is not protective against fracture in postmenopausal women: GLOW. Am. J. Med. 124, 1043–1050 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical