Infectious agents and neurodegeneration - PubMed (original) (raw)
Review
Infectious agents and neurodegeneration
Giovanna De Chiara et al. Mol Neurobiol. 2012 Dec.
Abstract
A growing body of epidemiologic and experimental data point to chronic bacterial and viral infections as possible risk factors for neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis. Infections of the central nervous system, especially those characterized by a chronic progressive course, may produce multiple damage in infected and neighbouring cells. The activation of inflammatory processes and host immune responses cause chronic damage resulting in alterations of neuronal function and viability, but different pathogens can also directly trigger neurotoxic pathways. Indeed, viral and microbial agents have been reported to produce molecular hallmarks of neurodegeneration, such as the production and deposit of misfolded protein aggregates, oxidative stress, deficient autophagic processes, synaptopathies and neuronal death. These effects may act in synergy with other recognized risk factors, such as aging, concomitant metabolic diseases and the host's specific genetic signature. This review will focus on the contribution given to neurodegeneration by herpes simplex type-1, human immunodeficiency and influenza viruses, and by Chlamydia pneumoniae.
Figures
Fig. 1
Infectious agents can reach the central nervous system by either crossing the blood–brain barrier (hematogen route) or being transported by axons of cranial nerve neurons (for further details, see text)
Fig. 2
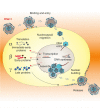
HSV is a double-stranded DNA virus, structurally composed of a linear genome packaged into an icosahedral capsid enclosed by tegument proteins and surrounded by a lipid bilayer membrane with embedded proteins and glycoproteins (envelope). Primary infection in humans usually occurs in the orofacial mucosa during childhood. There the virus replicates within the epithelial cells and undergoes its typical lytic life cycle ending with the production of infectious virions and lysis of the host cell. HSV entry into the host cell requires sequential interaction between specific viral membrane glycoproteins (gB, gC, gD, gH and gL) and cellular receptors [heparan sulphate proteoglycans (HSPG), nectin-1 and 2, herpesvirus entry mediator (HVEM) or 3-O sulphated heparan sulphate (3-OS HS)]. On entry, the nucleocapsid is transported to the nuclear membrane and the viral DNA is released into the nucleus for transcription of viral genes and replication. The HSV genome consists of two long structures of unique sequences (designated long (UL) and short (US)), that encode over 80 distinct genes, and it is transcribed by the RNA polymerase II of the infected host. Immediate-early genes are the first to be expressed following infection and they encode proteins that regulate the subsequent expression of early and late viral genes. Early gene expression then allows the synthesis of enzymes involved in DNA replication and the production of certain envelope glycoproteins. Expression of late genes occurs last; this group of genes predominantly encodes proteins that form the virus particle. This latter is then released from host cells by budding. HSV-1 is able to establish a lifelong latent infection in sensory neurons, particularly in cellular bodies of those feeding the site of primary infection [53]. This latency is characterized by the presence of a functional viral genome without production of the infectious virus. During this time, the latency-associated transcripts (LATs) are the only prominent transcripts [54] whose role in generating functional peptides or proteins is still a matter of debate [55]. Reactivation from latency can be triggered by several external stimuli (stress, immunosuppression, etc.) that activate viral gene expression. Newly produced virions are transported to the sites of primary infection where they cause recurrent herpetic lesions in some people. Interestingly, APOE4 is a risk factor for these recurrent herpetic lesions [56, 57]
Fig. 3
Chlamydiae are Gram-negative bacteria. They are obligate intracellular parasites because their multiplication depends on the host cell for energy and various nutrients. Chlamidiae have evolved a unique biphasic developmental cycle in which they alternate two distinct morphological forms: the elementary body (EB) and the reticulate body (RB). EBs are small tight bodies and represent the metabolically inactive form of bacteria, which can resist environmental stress and survive outside a host for a limited time. Infection begins with the attachment of the EB to the surface of susceptible host cells, followed by its internalization by endocytosis and the formation of phagosomes (_Chlamydi_a inclusions) that are heavily modified by chlamydial proteins which prevent their fusion with lysosomes. Shortly after uptake, EBs differentiate into the metabolically active form of RB and begin to replicate within the phagosomes. RBs replicate by binary fission that, after 24–72 h, becomes asynchronous, with some RBs converting back to EBs. Finally, EBs are released from infected cells, often after causing the death of the host cells, and can infect new cells, either in the same organism or in a new host. C. pneunoniae was classified as the third species of Chlamidia and was associated in humans with acute infections of the lower respiratory tract. It is recognized as a common cause of mild pneumonia in children and young adults. It infects both epithelial cells and macrophages within the lungs and may be disseminated to sites outside of the lungs by infected monocytes and macrophages. Infection may also persist or, alternatively, the bacterium may be present in asyntomatic patients. Recently, a large volume of research showed evidence that C. pneumoniae may contribute directly and indirectly (immuno-mediated) to atherosclerosis. Indeed, it was one of the few infectious agents that have been found within and isolated from cells of human atherosclerotic plaques
Fig. 4
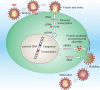
HIV-1 is an enveloped icosahedral retrovirus, belonging to the Lentivirus subgroup of Retroviridae family. Its genome is constituted by two identical copies of non-complementary positive single-stranded RNA, enclosed by a capsid composed of several copies of the viral protein p24. The single-stranded RNA is tightly bound to nucleocapsid proteins and enzymes needed for viral replication and assembly such as reverse transcriptase, proteases, ribonuclease and integrase. A matrix composed of the viral protein p17 surrounds the nucleocapsid and this is, in turn, surrounded by the viral envelope containing the surface glycoproteins gp120 and gp41 as protruding spikes. The HIV replication cycle begins with adsorption of the viral particles to CD4 molecules (a member of the immunoglobulin superfamily) on the surface of susceptible cells. The subsequent interaction with a co-receptor belonging to the family of chemokine receptors (CXCR4 or CCR5) plays a major role in membrane fusion and entry. Shortly after entry, subviral particles are partially uncoated in the cytoplasm and initiate the reverse transcription of viral RNA. The newly produced DNA is then transported into the nucleus and integrated into the host DNA by the virus-encoded integrase. The integrated HIV DNA is called provirus. The provirus may remain inactive for a long time, producing few or no new viral particles. The coordinated interaction of the HIV-encoded Tat protein and cellular transcription factors with the RNA polymerase II transcription apparatus starts the production of viral genomic RNA and messenger RNA, which is then spliced into smaller pieces, exported from the nucleus into the cytoplasm and translated into the proteins. In addition to viral structural proteins and enzymes, the HIV genome encodes the regulatory proteins (Rev and Tat, which is secreted by HIV-infected cells) and several accesory proteins: Vif, Vpu, Vpr, Vpx and Nef, playing an important role in the viral replication, disease pathogenesis and immune evasion. At the end of viral replication cycle, envelope polyproteins are transported to the plasma membrane where viral progeny begins assembly and budding from the infected cells. Then, subsequent proteolysis by viral protease generates mature particles
Fig. 5
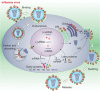
Influenza A viruses are enveloped, negative strand RNA viruses belonging to the Orthomyxoviridae family. Their genome consists of eight single-stranded RNA segments encoding 11 or 12 proteins: the receptor-binding haemagglutinin (HA); the sialic acid-destroying enzyme neuraminidase (NA), the ion channel M2, the matrix protein M1; the nucleoprotein (NP); the polymerase acidic protein (PA), polymerase basic proteins 1 and 2 (PB1, PB2) and the pro-apoptotic protein polymerase basic 1 (PB1)-F2; the nuclear export protein (NEP; also known as NS2) and the host antiviral response antagonist non-structural protein 1 (NS1); the newly identified N40 protein, which is expressed from the PB1 segment and has an unknown function [77]. Within the virion, each of the eight RNA segments forms a viral ribonucleoprotein (RNP) complex: in particular, viral RNA is wrapped around NP, and this structure is in turn bound to the viral polymerase complex, to constitute the viral nucleocapsid. In the initial stages of influenza A virus replication, the viral HA binds to host cell receptors that contain terminal α-2,6-linked or α-2,3-linked sialic acid (α-2,6-SA or α-2,3-SA) moieties, and the virus enters the cell by receptor-mediated endocytosis. Cleavage of HA by cellular proteases is required to expose the HA peptide that is responsible for the fusion between the viral envelope and the endosomal membrane. Acidification of the late endocytic vesicles allows the viral HA to undergo a conformational rearrangement that produces a fusogenic protein. The H+ ions in the acidic endosome are pumped, via the viral M2 ion channel, into the virus structure allowing the virus uncoating and the release of RNP complexes into the cytoplasm. The viral RNA is then imported in an ATP-dependent manner into the cell nucleus for transcription of genomic and messenger RNAs which are transported to the cytosol for translation. Viral HA, NA and M2 are synthesized in the Endoplasmic Reticulum, transported by the trans-Golgi secretory pathway and the mature proteins are inserted in the plasma membrane. New viral RNA is encased in the nucleocapsidic proteins and, together with matrix protein, is transported to cell surface where HA and NA will be incorporated. Progeny virions are then released from cells by budding
Fig. 6
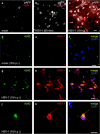
Effects of HSV-1 on APP phosphorylation and intracellular accumulation of amyloid-β protein (Aβ42) in cultured cortical neurons of E18 rats. a–c Time-dependent APP phosphorylation at threonine 668 induced by HSV-1; phosphorylated APP (pAPP) staining (white) in mock-infected cells (a), 1-h after cell challenge with HSV-1 (b) and 18 h post-infection (p.i.) (c). Neuronal infection is documented by HSV-1 labeling (red). d–f: no significant Aβ42 labeling (green) is observed in mock-infected cortical neurons. g–i Most HSV-1-infected neurons (red) exhibit Aβ42 immunoreactivity. j–l: high magnification image of a HSV-1-infected neuron showing marked Aβ42 staining. In f, i and l, the cell nuclei were stained with DAPI (blue). Calibration bars: 20 μm
Fig. 7
Molecular hallmarks of neurodegeneration induced by infectious agents in neurons of the central nervous system (for further details, see text)
Similar articles
- Infectious Agents and Neurodegenerative Diseases: Exploring the Links.
Alam MZ, Alam Q, Kamal MA, Jiman-Fatani AA, Azhar EI, Khan MA, Haque A. Alam MZ, et al. Curr Top Med Chem. 2017;17(12):1390-1399. doi: 10.2174/1568026617666170103164040. Curr Top Med Chem. 2017. PMID: 28049398 Review. - Redox Imbalance and Viral Infections in Neurodegenerative Diseases.
Limongi D, Baldelli S. Limongi D, et al. Oxid Med Cell Longev. 2016;2016:6547248. doi: 10.1155/2016/6547248. Epub 2016 Mar 27. Oxid Med Cell Longev. 2016. PMID: 27110325 Free PMC article. Review. - Molecular Mechanisms Associated with Neurodegeneration of Neurotropic Viral Infection.
Wongchitrat P, Chanmee T, Govitrapong P. Wongchitrat P, et al. Mol Neurobiol. 2024 May;61(5):2881-2903. doi: 10.1007/s12035-023-03761-6. Epub 2023 Nov 9. Mol Neurobiol. 2024. PMID: 37946006 Free PMC article. Review. - Infectious agents and age-related neurodegenerative disorders.
Mattson MP. Mattson MP. Ageing Res Rev. 2004 Jan;3(1):105-20. doi: 10.1016/j.arr.2003.08.005. Ageing Res Rev. 2004. PMID: 15163105 Free PMC article. Review. - Contribution of CNS and extra-CNS infections to neurodegeneration: a narrative review.
Kettunen P, Koistinaho J, Rolova T. Kettunen P, et al. J Neuroinflammation. 2024 Jun 6;21(1):152. doi: 10.1186/s12974-024-03139-y. J Neuroinflammation. 2024. PMID: 38845026 Free PMC article. Review.
Cited by
- Does Dementia Have a Microbial Cause?
Landry RL, Embers ME. Landry RL, et al. NeuroSci. 2022 May 17;3(2):262-283. doi: 10.3390/neurosci3020019. eCollection 2022 Jun. NeuroSci. 2022. PMID: 39483362 Free PMC article. Review. - Mycobacterium paratuberculosis: A HERV Turn-On for Autoimmunity, Neurodegeneration, and Cancer?
Dow CT, Pierce ES, Sechi LA. Dow CT, et al. Microorganisms. 2024 Sep 13;12(9):1890. doi: 10.3390/microorganisms12091890. Microorganisms. 2024. PMID: 39338563 Free PMC article. Review. - In silico modelling of neuron signal impact of cytokine storm-induced demyelination.
Adonias GL, Siljak H, Balasubramaniam S, Barros MT. Adonias GL, et al. Open Biol. 2024 Sep;14(9):240138. doi: 10.1098/rsob.240138. Epub 2024 Sep 4. Open Biol. 2024. PMID: 39226928 Free PMC article. - Microglia in Neurodegenerative Diseases.
Awogbindin I, Wanklin M, Verkhratsky A, Tremblay MÈ. Awogbindin I, et al. Adv Neurobiol. 2024;37:497-512. doi: 10.1007/978-3-031-55529-9_27. Adv Neurobiol. 2024. PMID: 39207709 Review. - Host response to Aplysia Abyssovirus 1 in nervous system and gill.
Kron NS, Fieber LA, Baker L, Campbell C, Schmale MC. Kron NS, et al. Dev Comp Immunol. 2024 Oct;159:105211. doi: 10.1016/j.dci.2024.105211. Epub 2024 Jun 15. Dev Comp Immunol. 2024. PMID: 38885747
References
- Bowery NG, Bagetta G, Nisticó G, et al. Intrahippocampal tetanus toxin produces generalized convulsions and neurodegeneration in rats: antagonism by NMDA receptor blockers. Epilepsy Res Suppl. 1992;9:249–256. - PubMed
- Cermelli C, Vinceti M, Beretti F, et al. Risk of sporadic amyotrophic lateral sclerosis associated with seropositivity for herpesvirus and echovirus-7. Eur J Epidemiol. 2003;18:123–127. - PubMed
- Ogata A, Tashiro K, Nukuzuma S, et al. A rat model of Parkinson's disease induced by Japanese encephalitis virus. J Neurovirol. 1997;3:141–147. - PubMed
- Menninger KA. Psychoses associated with influenza. Arch Neurol Psychiatr. 1919;2:291–337.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources