Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2 - PubMed (original) (raw)
. 2012 Aug 30;488(7413):652-5.
doi: 10.1038/nature11333.
Keiichi Inoue, Toru Yamashita, David B Rhee, Skylar Travis, Ryousuke Fujita, Paolo Guarnieri, Govind Bhagat, William B Vanti, Alan Shih, Ross L Levine, Sara Nik, Emily I Chen, Asa Abeliovich
Affiliations
- PMID: 22902501
- PMCID: PMC5176099
- DOI: 10.1038/nature11333
Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2
Claudia A Doege et al. Nature. 2012.
Abstract
Somatic cells can be reprogrammed into induced pluripotent stem cells (iPSCs) by using the pluripotency factors Oct4, Sox2, Klf4 and c-Myc (together referred to as OSKM). iPSC reprogramming erases somatic epigenetic signatures—as typified by DNA methylation or histone modification at silent pluripotency loci—and establishes alternative epigenetic marks of embryonic stem cells (ESCs). Here we describe an early and essential stage of somatic cell reprogramming, preceding the induction of transcription at endogenous pluripotency loci such as Nanog and Esrrb. By day 4 after transduction with OSKM, two epigenetic modification factors necessary for iPSC generation, namely poly(ADP-ribose) polymerase-1 (Parp1) and ten-eleven translocation-2 (Tet2), are recruited to the Nanog and Esrrb loci. These epigenetic modification factors seem to have complementary roles in the establishment of early epigenetic marks during somatic cell reprogramming: Parp1 functions in the regulation of 5-methylcytosine (5mC) modification, whereas Tet2 is essential for the early generation of 5-hydroxymethylcytosine (5hmC) by the oxidation of 5mC (refs 3,4). Although 5hmC has been proposed to serve primarily as an intermediate in 5mC demethylation to cytosine in certain contexts, our data, and also studies of Tet2-mutant human tumour cells, argue in favour of a role for 5hmC as an epigenetic mark distinct from 5mC. Consistent with this, Parp1 and Tet2 are each needed for the early establishment of histone modifications that typify an activated chromatin state at pluripotency loci, whereas Parp1 induction further promotes accessibility to the Oct4 reprogramming factor. These findings suggest that Parp1 and Tet2 contribute to an epigenetic program that directs subsequent transcriptional induction at pluripotency loci during somatic cell reprogramming.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
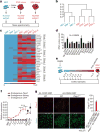
Figure 1. Parp1 promotes OSKM-mediated iPSC generation
a, Diagram of proteomic strategy to identify candidate epigenetic modification (EM) factors. b, Unsupervised hierarchical clustering analysis (Spearman rank correlation) of mass spectrometry data from nuclear extracts of MEFs (n = 3), iPSCs (n = 3) and ESCs (n = 1). The scale bar represents the correlation height (= 1 − Abs[correlation]). c, Dual-colour heat map for expression levels of 29 proteins highly enriched in both the iPSC and ESC samples (relative to MEFs). The colour scale bar represents the spectral count. Candidate EM factors were divided into six groups for further functional testing. d, Functional screen of candidate EM factors for promotion of somatic cell reprogramming in OSKM-MEFs. EM candidates were transduced together as a single pool of 29 genes, as 6 subpools, or as individual factors from group 6 (as in c). Alkaline phosphatase-positive (AP+) iPSC colonies were counted at day 14 after transduction with OSKM. e, Diagram of time-course analyses of iPSC reprogramming. f, Gene expression time course of endogenous Parp1, Nanog and Oct4. g, Immunocytochemistry analysis of WT or _Parp1_−/− d4-OSKM-MEFs and d4-CONT-MEFs with an antibody against Parp1 (upper panels; red), and counterstained with Sytox nuclear marker (lower panels; green). Increased Parp1 expression in d4-OSKM-MEFs is quantified on the right (Parp1HI; defined as mean plus 2 s.d. or greater than the expression level in d4-CONT-MEFs); modified nuclear morphology apparent in d4-OSKM-MEFs is as described previously. Results in d, f and g are shown as means and s.d. for three independent experiments. Asterisk, P < 0.05; three asterisks, P < 0.001.
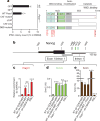
Figure 2. Parp1 activities during iPSC reprogramming
a, Functional analysis of Parp1 mutants for rescue of iPSC colony formation in _Parp1_−/− OSKM-MEFs. Cultures were transduced with green fluorescent protein (GFP), WT Parp1, or Parp1 mutants encoding a catalytic domain missense mutation (CAT mutant; E988K), deletion of the catalytic domain (ΔCAT), deletion of the DNA-binding and automodification domains (CAT-only), or triple missense mutation of the DNA-binding domain (DBD mutant; C21G/C125G/L139P). Zn, zinc fingers; BRCT, BRCA1 carboxy terminus. b, Schematic representation of the Nanog locus transcription start site (TSS) region. Indicated are _Hpa_II/_Msp_I sites (green bars) and amplicons for ‘exon 1/intron 1’ and ‘intron 1’ regions(thick greylines). bp,base pairs. c, Parp1 ChIP analyses of the cultures as indicated, presented as the relative enrichment to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). d, Content of 5hmC assessed by GlucMS-qPCR (as a percentage of total cytosine). e, Content of 5mC, quantified by subtraction of 5hmC content (as in d) from the total methylated cytosine (5mC + 5hmC, as determined by _Hpa_II digestion insensitivity; see Supplementary Fig. 3f). Results in a and c–e are shown as means and s.d. for three independent experiments. Asterisk, P < 0.05; two asterisks, P < 0.01; three asterisks, P < 0.001.
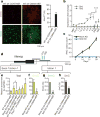
Figure 3. Tet2 is required for 5hmC formation at the Nanog locus
a, Immunocytochemistry of d4-OSKM-MEFs and d4-CONT-MEFs with an antibody against 5hmC (ref. 5) (upper panels; red) and counterstained with Sytox nuclear marker (lower panels; green). Representative images show increased 5hmC in d4-OSKM-MEFs, as quantified on the right (5hmCHI; defined as mean plus 2 s.d. or greater above the level in d4-CONT-MEFs). b, Time course of Tet1 and Tet2 gene expression assessed by qPCR (relative to ESC level). c, OSKM-mediated iPSC colony formation assay (AP+) in shRNA-mediated Tet2 knockdown (Tet2 KD; blue) and non-silencing control shRNA (mock KD; black)-treated MEFs. d, Diagram of the Nanog locus; regions are the same as in Fig. 2b. e, Tet2 ChIP-qPCR at the exon 1/intron 1 amplicon. f, g, Content of 5hmC in the cultures indicated, assessed by hMeDIP of the exon 1/intron 1 region (f, relative to GAPDH) or GlucMS-qPCR intron 1 amplicon (g, as a percentage of total cytosine). h, Content of 5mC at the intron 1 amplicon, quantified by subtraction of 5hmC (as in g) from the total methylated cytosine levels (as in Supplementary Fig. 4k; determined by _Hpa_II sensitivity assay; as a percentage of total cytosine). Results in a–c and e–h are shown as means and s.d. for three independent experiments. Asterisk, P < 0.05; two asterisks, P < 0.01; three asterisks, P < 0.001.
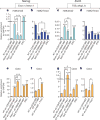
Figure 4. Impact of Parp1 and Tet2 on chromatin state and Oct4 accessibility at the Nanog and Esrrb loci
a–d, H3K4me2 (a, c) and H3K27me3 (b, d) ChIP-qPCR at Nanog or Esrrb amplicons in cultures as indicated. e–h, Oct4 ChIP-qPCR. Results are shown as means and s.d. for three independent experiments. Asterisk, P < 0.05; two asterisks, P < 0.01; three asterisks, P < 0.001.
Comment in
- Epigenetics: Actors in the cell reprogramming drama.
Loh KM, Lim B. Loh KM, et al. Nature. 2012 Aug 30;488(7413):599-600. doi: 10.1038/488599a. Nature. 2012. PMID: 22932382 No abstract available.
Similar articles
- Artd1/Parp1 regulates reprogramming by transcriptional regulation of Fgf4 via Sox2 ADP-ribosylation.
Weber FA, Bartolomei G, Hottiger MO, Cinelli P. Weber FA, et al. Stem Cells. 2013 Nov;31(11):2364-73. doi: 10.1002/stem.1507. Stem Cells. 2013. PMID: 23939864 - Poly(ADP-ribose) polymerase 1 regulates nuclear reprogramming and promotes iPSC generation without c-Myc.
Chiou SH, Jiang BH, Yu YL, Chou SJ, Tsai PH, Chang WC, Chen LK, Chen LH, Chien Y, Chiou GY. Chiou SH, et al. J Exp Med. 2013 Jan 14;210(1):85-98. doi: 10.1084/jem.20121044. Epub 2012 Dec 31. J Exp Med. 2013. PMID: 23277454 Free PMC article. - NANOG-dependent function of TET1 and TET2 in establishment of pluripotency.
Costa Y, Ding J, Theunissen TW, Faiola F, Hore TA, Shliaha PV, Fidalgo M, Saunders A, Lawrence M, Dietmann S, Das S, Levasseur DN, Li Z, Xu M, Reik W, Silva JC, Wang J. Costa Y, et al. Nature. 2013 Mar 21;495(7441):370-4. doi: 10.1038/nature11925. Epub 2013 Feb 10. Nature. 2013. PMID: 23395962 Free PMC article. - Transcriptional and epigenetic mechanisms of cellular reprogramming to induced pluripotency.
van den Hurk M, Kenis G, Bardy C, van den Hove DL, Gage FH, Steinbusch HW, Rutten BP. van den Hurk M, et al. Epigenomics. 2016 Aug;8(8):1131-49. doi: 10.2217/epi-2016-0032. Epub 2016 Jul 15. Epigenomics. 2016. PMID: 27419933 Free PMC article. Review. - Poly(ADP-Ribose) Polymerase 1: Cellular Pluripotency, Reprogramming, and Tumorogenesis.
Jiang BH, Tseng WL, Li HY, Wang ML, Chang YL, Sung YJ, Chiou SH. Jiang BH, et al. Int J Mol Sci. 2015 Jul 9;16(7):15531-45. doi: 10.3390/ijms160715531. Int J Mol Sci. 2015. PMID: 26184161 Free PMC article. Review.
Cited by
- Crosstalk Between DNA and Histones: Tet's New Role in Embryonic Stem Cells.
Sui X, Price C, Li Z, Chen J. Sui X, et al. Curr Genomics. 2012 Dec;13(8):603-8. doi: 10.2174/138920212803759730. Curr Genomics. 2012. PMID: 23730200 Free PMC article. - Mechanisms and models of somatic cell reprogramming.
Buganim Y, Faddah DA, Jaenisch R. Buganim Y, et al. Nat Rev Genet. 2013 Jun;14(6):427-39. doi: 10.1038/nrg3473. Nat Rev Genet. 2013. PMID: 23681063 Free PMC article. Review. - DNA double-strand breaks coupled with PARP1 and HNRNPA2B1 binding sites flank coordinately expressed domains in human chromosomes.
Tchurikov NA, Kretova OV, Fedoseeva DM, Sosin DV, Grachev SA, Serebraykova MV, Romanenko SA, Vorobieva NV, Kravatsky YV. Tchurikov NA, et al. PLoS Genet. 2013 Apr;9(4):e1003429. doi: 10.1371/journal.pgen.1003429. Epub 2013 Apr 4. PLoS Genet. 2013. PMID: 23593027 Free PMC article. - Breathing-in epigenetic change with vitamin C.
Monfort A, Wutz A. Monfort A, et al. EMBO Rep. 2013 Apr;14(4):337-46. doi: 10.1038/embor.2013.29. Epub 2013 Mar 15. EMBO Rep. 2013. PMID: 23492828 Free PMC article. Review. - Manipulating cell fate through reprogramming: approaches and applications.
Yagi M, Horng JE, Hochedlinger K. Yagi M, et al. Development. 2024 Oct 1;151(19):dev203090. doi: 10.1242/dev.203090. Epub 2024 Sep 30. Development. 2024. PMID: 39348466 Review.
References
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1S10RR023680-1/RR/NCRR NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- S10 RR023680/RR/NCRR NIH HHS/United States
- R01 NS064433/NS/NINDS NIH HHS/United States
- R01 CA173636/CA/NCI NIH HHS/United States
- R01 138424/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous