Single reconstituted neuronal SNARE complexes zipper in three distinct stages - PubMed (original) (raw)
Single reconstituted neuronal SNARE complexes zipper in three distinct stages
Ying Gao et al. Science. 2012.
Abstract
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins drive membrane fusion by assembling into a four-helix bundle in a zippering process. Here, we used optical tweezers to observe in a cell-free reconstitution experiment in real time a long-sought SNARE assembly intermediate in which only the membrane-distal amino-terminal half of the bundle is assembled. Our findings support the zippering hypothesis, but suggest that zippering proceeds through three sequential binary switches, not continuously, in the amino- and carboxyl-terminal halves of the bundle and the linker domain. The half-zippered intermediate was stabilized by externally applied force that mimicked the repulsion between apposed membranes being forced to fuse. This intermediate then rapidly and forcefully zippered, delivering free energy of 36 k(B)T (where k(B) is Boltzmann's constant and T is temperature) to mediate fusion.
Figures
Fig. 1
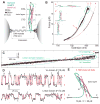
Dynamic disassembly and reassembly of the single SNARE complex. (A) Experimental setup. The SNARE complex contains the N-terminal (NTD) and C-terminal (CTD) SNARE domains, with the corresponding VAMP2 regions designed as Vn and Vc, respectively, separated by the ionic layer and the linker domain (LD). (B) Force-extension curve (FEC) of the SNARE-DNA conjugate. The FEC corresponds to the first of 5 cycles of pull (black) and relaxation (gray) shown in fig. S2. Different segments of the FEC can be fit by the worm-like chain model (red dashed lines), revealing the structures of SNARE assembly states (inset, same red numbering throughout the figures). The LD and CTD transitions are marked by dashed and solid ovals, respectively. (C) Time-dependent extension corresponding to the pulling phase from 8.6 pN to 17.5 pN (fig. S3). (D) Extension transitions of LD (bottom panel) and CTD (top panel) with their idealized transitions determined by the HMM analysis (red traces). The histogram distributions of extension are shown in fig. S5. (E) Structural model of the force-dependent half-zippered state with Vc unzipped to the ionic layer (red).
Fig. 2
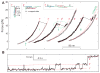
SNAP-25-dependent SNARE assembly. (A) Force-dependent unzipping probability of CTD measured on a single SNARE complex. (B) Corresponding CTD transition rates. Theoretical predications are shown in lines. (C) FECs measured in the absence (− SNAP-25) and presence (+ SNAP-25) of SNAP-25 in solution.
Fig. 3
Disassembly and reassembly of the SNARE complex under N-terminal pulling force. (A) FECs and their segmental fit (red dashed line) showing different assembly states (inset) including the completely unfolded state 4′. The full SNARE reassembly (cyan arrow) is t-SNARE dependent. (B) Extension-time trace corresponding to the region marked in the dashed oval in A.
Fig. 4
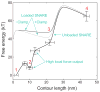
Sketches of the energy landscapes for SNARE zippering in the absence (black) and presence of the opposing force load from membranes with (light gray) and without (gray) complexin clamp. The contour length of the SNARE complex between the C-termini of syntaxin (residue 265) and VAMP2 (residue 92) is chosen as a reaction coordinate. Error bars show the standard deviations of the measurements.
Comment in
- Cell biology. Staging membrane fusion.
Rizo J. Rizo J. Science. 2012 Sep 14;337(6100):1300-1. doi: 10.1126/science.1228654. Science. 2012. PMID: 22984057 No abstract available.
Similar articles
- Stability, folding dynamics, and long-range conformational transition of the synaptic t-SNARE complex.
Zhang X, Rebane AA, Ma L, Li F, Jiao J, Qu H, Pincet F, Rothman JE, Zhang Y. Zhang X, et al. Proc Natl Acad Sci U S A. 2016 Dec 13;113(50):E8031-E8040. doi: 10.1073/pnas.1605748113. Epub 2016 Nov 28. Proc Natl Acad Sci U S A. 2016. PMID: 27911771 Free PMC article. - Common intermediates and kinetics, but different energetics, in the assembly of SNARE proteins.
Zorman S, Rebane AA, Ma L, Yang G, Molski MA, Coleman J, Pincet F, Rothman JE, Zhang Y. Zorman S, et al. Elife. 2014 Sep 1;3:e03348. doi: 10.7554/eLife.03348. Elife. 2014. PMID: 25180101 Free PMC article. - Energetics, kinetics, and pathway of SNARE folding and assembly revealed by optical tweezers.
Zhang Y. Zhang Y. Protein Sci. 2017 Jul;26(7):1252-1265. doi: 10.1002/pro.3116. Epub 2017 Mar 8. Protein Sci. 2017. PMID: 28097727 Free PMC article. Review. - A half-zippered SNARE complex represents a functional intermediate in membrane fusion.
Li F, Kümmel D, Coleman J, Reinisch KM, Rothman JE, Pincet F. Li F, et al. J Am Chem Soc. 2014 Mar 5;136(9):3456-64. doi: 10.1021/ja410690m. Epub 2014 Feb 18. J Am Chem Soc. 2014. PMID: 24533674 Free PMC article. - SNARE zippering.
Lou X, Shin YK. Lou X, et al. Biosci Rep. 2016 May 6;36(3):e00327. doi: 10.1042/BSR20160004. Print 2016 Jun. Biosci Rep. 2016. PMID: 27154457 Free PMC article. Review.
Cited by
- Complexin splits the membrane-proximal region of a single SNAREpin.
Yin L, Kim J, Shin YK. Yin L, et al. Biochem J. 2016 Jul 15;473(14):2219-24. doi: 10.1042/BCJ20160339. Epub 2016 May 24. Biochem J. 2016. PMID: 27222590 Free PMC article. - Synaptotagmin-7 places dense-core vesicles at the cell membrane to promote Munc13-2- and Ca2+-dependent priming.
Tawfik B, Martins JS, Houy S, Imig C, Pinheiro PS, Wojcik SM, Brose N, Cooper BH, Sørensen JB. Tawfik B, et al. Elife. 2021 Mar 22;10:e64527. doi: 10.7554/eLife.64527. Elife. 2021. PMID: 33749593 Free PMC article. - Synaptic-vesicle fusion: a need for speed.
Munson M. Munson M. Nat Struct Mol Biol. 2015 Jul;22(7):509-11. doi: 10.1038/nsmb.3056. Nat Struct Mol Biol. 2015. PMID: 26150331 No abstract available. - Sec17/Sec18 can support membrane fusion without help from completion of SNARE zippering.
Song H, Torng TL, Orr AS, Brunger AT, Wickner WT. Song H, et al. Elife. 2021 May 4;10:e67578. doi: 10.7554/eLife.67578. Elife. 2021. PMID: 33944780 Free PMC article. - Mechanical unzipping and rezipping of a single SNARE complex reveals hysteresis as a force-generating mechanism.
Min D, Kim K, Hyeon C, Cho YH, Shin YK, Yoon TY. Min D, et al. Nat Commun. 2013;4:1705. doi: 10.1038/ncomms2692. Nat Commun. 2013. PMID: 23591872 Free PMC article.
References
- Sollner T, et al. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318. - PubMed
- Weber T, et al. SNAREpins: Minimal machinery for membrane fusion. Cell. 1998;92:759. - PubMed
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 angstrom resolution. Nature. 1998;395:347. - PubMed
- Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK027044/DK/NIDDK NIH HHS/United States
- R37 DK027044/DK/NIDDK NIH HHS/United States
- R01 GM093341/GM/NIGMS NIH HHS/United States
- DK027044/DK/NIDDK NIH HHS/United States
- GM093341/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources