Autistic-like behaviour in Scn1a+/- mice and rescue by enhanced GABA-mediated neurotransmission - PubMed (original) (raw)
. 2012 Sep 20;489(7416):385-90.
doi: 10.1038/nature11356. Epub 2012 Aug 22.
Affiliations
- PMID: 22914087
- PMCID: PMC3448848
- DOI: 10.1038/nature11356
Autistic-like behaviour in Scn1a+/- mice and rescue by enhanced GABA-mediated neurotransmission
Sung Han et al. Nature. 2012.
Abstract
Haploinsufficiency of the SCN1A gene encoding voltage-gated sodium channel Na(V)1.1 causes Dravet's syndrome, a childhood neuropsychiatric disorder including recurrent intractable seizures, cognitive deficit and autism-spectrum behaviours. The neural mechanisms responsible for cognitive deficit and autism-spectrum behaviours in Dravet's syndrome are poorly understood. Here we report that mice with Scn1a haploinsufficiency exhibit hyperactivity, stereotyped behaviours, social interaction deficits and impaired context-dependent spatial memory. Olfactory sensitivity is retained, but novel food odours and social odours are aversive to Scn1a(+/-) mice. GABAergic neurotransmission is specifically impaired by this mutation, and selective deletion of Na(V)1.1 channels in forebrain interneurons is sufficient to cause these behavioural and cognitive impairments. Remarkably, treatment with low-dose clonazepam, a positive allosteric modulator of GABA(A) receptors, completely rescued the abnormal social behaviours and deficits in fear memory in the mouse model of Dravet's syndrome, demonstrating that they are caused by impaired GABAergic neurotransmission and not by neuronal damage from recurrent seizures. These results demonstrate a critical role for Na(V)1.1 channels in neuropsychiatric functions and provide a potential therapeutic strategy for cognitive deficit and autism-spectrum behaviours in Dravet's syndrome.
Figures
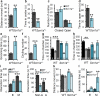
Figure 1. Scn1a+/− mice display hyperactivity, anxiety-like behavior, increased stereotypies, poor nest-building, and impaired social behavior
In the open field test, Scn1a+/− mice travel longer distances compared with WT mice (a), spend less time in the center (b), spend more time grooming (e) and circling (f) than WT mice. In the elevated plus maze, Scn1a+/− mice enter less frequently in the open arms (c) and spend less time in the open arms (d). In (f) one complete turn is counted as one circle, regardless of direction. g, h, Three-chamber experiment. g, Whereas WT mice spend more time in the chamber housing a stranger mouse (M) than the chamber housing an empty cage (E), Scn1a+/− mice have no preference for either chamber. h, Whereas WT mice spend more time in the chamber housing a novel mouse (M2) than in a chamber housing a familiar mouse (M1), Scn1a+/− mice have no preference for either chamber. i - l, Social interaction test. i, Scn1a+/− mice show decreased interaction with a caged stranger mouse when compared with WT mice. j, In a 10-min reciprocal interaction test, pairs of WT and Scn1a+/− unfamiliar mice had significantly less non-aggressive (Non-A) and aggressive (A) interactions than pairs of WT and WT unfamiliar mice. Aggressive behaviors included attacking, wrestling, and biting the dorsal surface, and non-aggressive behaviors include nose-to-nose sniffing, anogenital sniffing, and grooming. All data shown are means ± s.e.m. from 10 – 12 mice per genotype. *P < 0.05, **P < 0.01, ***P < 0.001. k, Scn1a+/− mice move significantly less when they encountered the stranger mouse compared to an empty cage, whereas there is no difference in movement for WT. l, Scn1a+/− mice, but not WT mice, show increased immobilization behavior in the presence of the caged stranger mouse than in the presence of an empty cage.
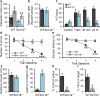
Figure 2. Profound deficits in context-dependent spatial learning and memory formation in Scn1a+/− mice
a, In the novel object recognition test, Scn1a+/− mice show normal recognition memory for preconditioned object (F: Familiar), which was presented 24 hr before the test, so that they spend more time with novel object (N: Novel). b, Discrimination index, the normalized ratio of time spent with the familiar object and time spent with the novel object, shows that there is no difference between WT and Scn1a+/− mice for novel object recognition ability. c, In the contextual fear conditioning test, Scn1a+/− mice display a profound deficits in short-term (30 min) and long-term (24 hr) memory of 2 s mild foot shock (0.5 mA)-associated context, but normal fear response immediately after the training (Train) when compared to WT mice. d, e, In the Barnes circular maze test, Scn1a+/− mice display profound deficit in spatial learning. WT mice make less errors to find the target hole (d), and display decreased latency to escape the maze (e) during the 4-day repeated training trials, but Scn1a+/− mice display no improved performance for both the number of errors made to find the target hole (d), and the time to escape the maze (e). f - i, During the probe trial at 5th day of trials, Scn1a+/− mice display profound deficit in spatial memory. They spend significantly more time to find the target hole (g), poke target hole with significantly lower correct choice (h), and stay significantly less time in the target area (i) when compared with WT mice, although total moved distance is not significantly different with that of WT mice (f). All data shown are means ± s.e.m. from 6 – 10 mice per genotype. *P < 0.05, #, ***P < 0.001.
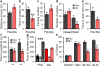
Figure 3. Dlx1/2-Scn1a+/− mice have the impaired spatial learning and autism-related phenotypes observed in Scn1a+/− mice
a, In the open field test, Dlx1/2-Scn1a+/− mice run longer compared with Cre-negative floxed littermate mice. b, In the open field test, Dlx1/2-Scn1a+/− mice spend less time in the center during the open field test. c, Dlx1/2-Scn1a+/− mice show increased circling behavior. One complete turn, regardless of direction is counted as one circling. d, e, In the elevated plus maze, Dlx1/2-Scn1a+/− mice enter less frequently in open arms (d), and spend significantly less time in open arms (e). f, In the social interaction test, Dlx1/2-Scn1a+/− mice display decreased interaction with social cue when compared to negative floxed littermate mice. g , In the 3-chamber test, Dlx1/2-Scn1a+/− mice have no preference for the stranger mouse. h, In the contextual fear conditioning test, Dlx1/2-Scn1a+/− mice display profound deficit in short-term (30 min) and long-term (24 hr) memory of 2 s mild foot shock (0.5 mA)-associated context, but normal fear response immediately after the foot shock during training (Train) when compared to WT mice. Dlx, Dlx1/2-Scn1a+/− mice. Flox, Cre-negative floxed Scn1a+/+ mice. E, Empty cage. C, Center. M, Mouse. All data shown are means ± s.e.m. from 7 – 9 mice per genotype. *P < 0.05; **P < 0.01; #, ***P < 0.001.
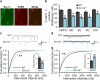
Figure 4. Deficit of NaV1.1 channels and GABAergic neurotransmission in Scn1a+/− hippocampal GABAergic interneurons
Immunocytochemical staining of forebrain neurons from 10-month old mice for NaV1.1 channels. a, Co-immunolabeling of NaV1.1 and GABA reveals co-expression of NaV1.1 and GABA in the hippocampal CA1 region in WT mice. b, Co-immunolabeling of NaV1.1 and GABA reveals a decreased expression of NaV1.1 channels in GABAergic interneurons in various forebrain regions in Scn1a+/− mice. c, Example traces of sIPSC from WT and Scn1a+/− mice hippocampal CA1 region. d, Example traces of sEPSC from WT and Scn1a+/− mice hippocampal CA1 region. f, Cumulative plot and average values (inset) of sIPSC frequency. The frequency of sIPSC is decreased, but the amplitude of sIPSC is unchanged in Scn1a+/− hippocampal CA1 slices when compared to WT slices (Suppl. Fig. 17a). g, Cumulative plot and average values (inset) of sEPSC frequency. The frequency of sEPSC is increased, but the amplitude of sEPSC is unchanged in Scn1a+/− hippocampal CA1 slices when compared to WT slices (Suppl. Fig. 17b). mPFC, medial prefrontal cortex. MC, motor cortex. SC, sensory cortex. PC, parietal cortex. CA1, hippocampal CA1 region. All data shown are means ± s.e.m. from 15 – 19 recordings per genotype. #, **P < 0.01.
Figure 5. Complete rescue of impaired social behavior and fear-associated memory deficits by low-dose clonazepam treatment
a, Both WT and Scn1a+/− mice show dose-dependent sedation by CLZ. Maximal concentration of CLZ without sedative effect is 0.0625 mg/kg. b, c, In the social interaction test (b) and 3-chamber test (c), decreased social interaction in Scn1a+/− mice is completely recovered by a single i.p. injection of 0.0625 mg/kg CLZ, 30 min prior to the test; this CLZ effect on social interaction completely disappears after 1 week of clearance period in the same Scn1a+/− mice. CLZ effects on social interaction are absent in WT mice. d, e, In the contextual fear conditioning test, a single i.p. injection of 0.0625 mg/kg CLZ, 30 min prior to the training, leads to a complete rescue of short-term (30 min) and long-term (24 h) fear-associated contextual memory in Scn1a+/− mice (e), but no significant change of fear-associated contextual memory by CLZ is observed in WT mice (d). Pre, Pre-clonazepam treated. CLZ, Clonazepam. Post, Post-Clonazepam treated. All data shown are means ± s.e.m. from 6 – 12 mice per genotype. ns, not significant. f, Cumulative plot and average value (inset) of sIPSC amplitude. The treatment of 10 μM CLZ increases the amplitude of sIPSC, but the frequency of sIPSC is unchanged by 10 μM CLZ in Scn1a+/− hippocampal CA1 slices (Suppl. Fig. 24a). g, Cumulative plot and average value (inset) of sEPSC frequency. The treatment of 10 μM CLZ decreases the frequency of sEPSC, but the amplitude of sEPSC is unchanged by 10 μM CLZ in Scn1a+/− hippocampal CA1 slices (Suppl. Fig. 24b). All data shown are means ± s.e.m. from 15 – 20 recordings per treatment group. *P < 0.05, **P < 0.01, ***P < 0.001.
Similar articles
- Dissecting the phenotypes of Dravet syndrome by gene deletion.
Rubinstein M, Han S, Tai C, Westenbroek RE, Hunker A, Scheuer T, Catterall WA. Rubinstein M, et al. Brain. 2015 Aug;138(Pt 8):2219-33. doi: 10.1093/brain/awv142. Epub 2015 May 27. Brain. 2015. PMID: 26017580 Free PMC article. - Clonazepam attenuates neurobehavioral abnormalities in offspring exposed to maternal immune activation by enhancing GABAergic neurotransmission.
Yang Y, Wang B, Zhong Z, Chen H, Ding W, Hoi MPM. Yang Y, et al. Biochem Pharmacol. 2021 Oct;192:114711. doi: 10.1016/j.bcp.2021.114711. Epub 2021 Jul 26. Biochem Pharmacol. 2021. PMID: 34324871 - Synergistic GABA-enhancing therapy against seizures in a mouse model of Dravet syndrome.
Oakley JC, Cho AR, Cheah CS, Scheuer T, Catterall WA. Oakley JC, et al. J Pharmacol Exp Ther. 2013 May;345(2):215-24. doi: 10.1124/jpet.113.203331. Epub 2013 Feb 19. J Pharmacol Exp Ther. 2013. PMID: 23424217 Free PMC article. - NaV1.1 channels and epilepsy.
Catterall WA, Kalume F, Oakley JC. Catterall WA, et al. J Physiol. 2010 Jun 1;588(Pt 11):1849-59. doi: 10.1113/jphysiol.2010.187484. Epub 2010 Mar 1. J Physiol. 2010. PMID: 20194124 Free PMC article. Review. - Debate: Does genetic information in humans help us treat patients? PRO--genetic information in humans helps us treat patients. CON--genetic information does not help at all.
Delgado-Escueta AV, Bourgeois BF. Delgado-Escueta AV, et al. Epilepsia. 2008 Dec;49 Suppl 9:13-24. doi: 10.1111/j.1528-1167.2008.01922.x. Epilepsia. 2008. PMID: 19087113 Review.
Cited by
- Both GEF domains of the autism and developmental epileptic encephalopathy-associated Trio protein are required for proper tangential migration of GABAergic interneurons.
Eid L, Lokmane L, Raju PK, Tene Tadoum SB, Jiang X, Toulouse K, Lupien-Meilleur A, Charron-Ligez F, Toumi A, Backer S, Lachance M, Lavertu-Jolin M, Montseny M, Lacaille JC, Bloch-Gallego E, Rossignol E. Eid L, et al. Mol Psychiatry. 2024 Sep 19. doi: 10.1038/s41380-024-02742-y. Online ahead of print. Mol Psychiatry. 2024. PMID: 39300136 - The Biallelic Inheritance of Two Novel SCN1A Variants Results in Developmental and Epileptic Encephalopathy Responsive to Levetiracetam.
Dinoi G, Conte E, Palumbo O, Benvenuto M, Coppola MA, Palumbo P, Lastella P, Boccanegra B, Di Muro E, Castori M, Carella M, Sciruicchio V, de Tommaso M, Liantonio A, De Luca A, La Neve A, Imbrici P. Dinoi G, et al. Biomedicines. 2024 Jul 31;12(8):1698. doi: 10.3390/biomedicines12081698. Biomedicines. 2024. PMID: 39200163 Free PMC article. - Epigenetic insights into GABAergic development in Dravet Syndrome iPSC and therapeutic implications.
Schuster J, Lu X, Dang Y, Klar J, Wenz A, Dahl N, Chen X. Schuster J, et al. Elife. 2024 Aug 27;12:RP92599. doi: 10.7554/eLife.92599. Elife. 2024. PMID: 39190448 Free PMC article. - An alpha 5-GABAA receptor positive allosteric modulator attenuates social and cognitive deficits without changing dopamine system hyperactivity in rats exposed to valproic acid in utero.
Souza AJ, Freitas ÍS, Sharmin D, Cook JM, Guimarães FS, Gomes FV. Souza AJ, et al. Autism Res. 2024 Aug;17(8):1534-1544. doi: 10.1002/aur.3178. Epub 2024 Jun 22. Autism Res. 2024. PMID: 39169698 - Axo-axonic synaptic input drives homeostatic plasticity by tuning the axon initial segment structurally and functionally.
Zhao R, Ren B, Xiao Y, Tian J, Zou Y, Wei J, Qi Y, Hu A, Xie X, Huang ZJ, Shu Y, He M, Lu J, Tai Y. Zhao R, et al. Sci Adv. 2024 Aug 2;10(31):eadk4331. doi: 10.1126/sciadv.adk4331. Epub 2024 Aug 2. Sci Adv. 2024. PMID: 39093969 Free PMC article.
References
- Wolff M, Casse-Perrot C, Dravet C. Severe myoclonic epilepsy of infants (Dravet syndrome): natural history and neuropsychological findings. Epilepsia. 2006;47(Suppl 2):45–48. - PubMed
- Genton P, Velizarova R, Dravet C. Dravet syndrome: the long-term outcome. Epilepsia. 2011;52(Suppl 2):44–49. - PubMed
- Li BM, et al. Autism in Dravet syndrome: prevalence, features, and relationship to the clinical characteristics of epilepsy and mental retardation. Epilepsy Behav. 2011;21:291–295. - PubMed
- Brunklaus A, Dorris L, Zuberi SM. Comorbidities and predictors of health-related quality of life in Dravet syndrome. Epilepsia. 2011;52:1476–1482. - PubMed
- Besag FM. Behavioral aspects of pediatric epilepsy syndromes. Epilepsy Behav. 2004;5(Suppl 1):S3–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH075016/MH/NIMH NIH HHS/United States
- R01 NS025704/NS/NINDS NIH HHS/United States
- R37 MH049428/MH/NIMH NIH HHS/United States
- R01 NS25704/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases