Intranasal treatment with poly(I•C) protects aged mice from lethal respiratory virus infections - PubMed (original) (raw)
Intranasal treatment with poly(I•C) protects aged mice from lethal respiratory virus infections
Jincun Zhao et al. J Virol. 2012 Nov.
Abstract
In the 2002-2003 severe acute respiratory syndrome coronavirus (SARS-CoV) epidemic, no patients under 24 years of age died, while mortality was greater than 50% in those over 65 years. Greater than 90% of all deaths from influenza A virus (IAV) occur in the elderly (>65 years of age). To address this age-related susceptibility to SARS-CoV and IAV, we infected C57BL/6 (B6) mice with mouse-adapted SARS-CoV (MA15) or IAV (PR8), both of which cause severe disease in aged mice. Intranasal pretreatment of aged mice with poly(I·C) (a TLR3 agonist) and, to a lesser extent, CpG, R848, or lipopolysaccharide (TLR9, TLR7/8, or TLR4 agonists), provided a high level of protection [90% to 100% survival rate after poly(I·C) treatment] against lethal MA15 or IAV challenge and reduced pathological changes and virus loads in the lungs at early times after infection. Poly(I·C) pretreatment upregulated beta interferon (IFN-β), IFN-γ, IL-1β, and tumor necrosis factor (TNF) gene expression in the lungs. Intranasal pretreatment with IFN-β or IFN-γ but not IL-1β or TNF also protected aged mice, consistent with the notion that poly(I·C) pretreatment functioned, at least in part, by inducing IFN-β and IFN-γ. We also identified a potential cellular target for poly(I·C) by showing that treatment inhibited virus replication in primary human airway epithelial cells. These results suggest that intranasal poly(I·C) should be evaluated as a prophylactic agent in aged individuals at high risk for contracting SARS-CoV or IAV infections.
Figures
Fig 1
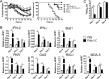
Effect of TLR agonist treatments on weight loss, virus titers, mortality, and histological changes in MA15-infected aged B6 mice. (A) Twelve-month-old B6 mice were treated with 20 μg poly(I·C), 100 μg CpG, 5 μg LPS, 10 μg R848, or PBS 6 h before intranasal infection with 1 × 105 PFU MA15 virus. Weight loss and mortality were monitored daily. n = 17 mice in the PBS group, 13 in the poly(I·C) group, 6 in the LPS group, 7 in the CpG group, and 8 in R848 group. (B) To obtain virus titers, lungs were homogenized and virus titers determined on Vero E6 cells. Titers are expressed as PFU/g tissue. n = 4 mice/group/time point. Data are representative of 2 independent experiments. *, P < 0.05. (C) Twenty-two-month-old B6 mice were treated with 20 μg poly(I·C) or PBS 6 h prior to infection with 1 × 105 PFU MA15 virus. n = 7 mice in the PBS group and 8 mice in the poly(I·C) group. (D) Human polarized airway epithelial cells were treated with poly(I·C) (1 μg/ml) and infected with MA15 as described in Materials and Methods. Virus titers were determined by plaque assay. Data are representative of 3 independent experiments.
Fig 2
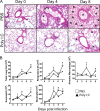
Poly(I·C) treatment reduces pulmonary edema and accelerates inflammatory cell infiltration into SARS-CoV-infected lungs. Twelve-month-old B6 mice were treated with 20 μg poly(I·C) or PBS 6 h before intranasal infection with 1 × 105 PFU MA15 virus. (A) Lungs were removed at the indicated time points p.i., fixed in zinc formalin, and embedded in paraffin. Sections were stained with hematoxylin and eosin. Asterisks indicate edema, arrowhead shows a foamy macrophage, and arrows indicate marginating cells. (B) Necrotic debris (arrows) was detected at day 2 p.i. in the airways of PBS-treated mice. (C) Lungs were harvested at the indicated time points, and after enzyme digestion, single-cell suspensions were acquired. Total numbers of lung cells, rDCs (CD11c+ MHCII+ SiglecF−), alveolar macrophages (CD11c+ MHCII+/− SiglecF+), eosinophils (CD45+ CD11c+/− SiglecF+), and neutrophils (CD11b+ Ly6C+ Ly6G+) in the lung are shown. Data are representative of 2 independent experiments, n = 4 mice/group/time point. *, P < 0.05.
Fig 3
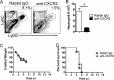
Blocking neutrophil migration to the lungs did not protect 12-month-old mice from lethal SARS-CoV. Twelve-month-old mice were treated with 250 μg rabbit anti-CXCR2 polyclonal antibodies i.p. at days −3 and 0 and at day 3 after intranasal infection with 1 × 105 PFU MA15 virus. Lung cells were harvested 2 days after SARS-CoV infection. Neutrophil frequency (A) and numbers (B) are shown. Data are representative of 3 independent experiments. *, P < 0.05. Weight loss (C) and mortality (D) were monitored daily. n = 6 mice in rabbit IgG and anti-CXCR2 groups.
Fig 4
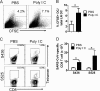
Poly(I·C) treatment enhances rDC migration and T cell responses in the lungs of aged mice. (A and B) Twelve-month-old mice were treated with poly(I·C) or PBS 2 h before intranasal inoculation of 50 μl 8 mM CFSE. Four h after CFSE instillation, mice were infected with 1 × 105 PFU MA15 virus. At 18 h p.i., single-cell suspensions were prepared from lung DLNs and gated for CD11c and MHC-II expression by flow cytometry. The values represent the percentage of CFSE+ cells within the CD11c+ MHC-II+ DC population. (B and D) Lung cells were harvested from 12-month-old B6 mice 6 days after SARS-CoV infection. Tetramer staining for CD8 T cell epitopes S436 and S525 is shown. n = 4 mice/group/time point. Data are representative of 3 independent experiments.
Fig 5
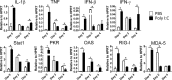
Cytokine and chemokine RNA levels in 12-month-old mice after poly(I·C) treatment and SARS-CoV infection. Mice were treated with poly(I·C) 6 h prior to infection with MA15. RNA was extracted from MA15-infected lungs at the indicated time points and processed as described in Materials and Methods. n = 4 mice/group/experiment. Data are representative of 2 independent experiments. *, P < 0.05. HPRT, hypoxanthine phosphoribosyltransferase.
Fig 6
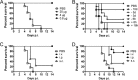
Effect of cytokine or TLR agonist treatments on weight loss and mortality in MA15-infected aged B6, IFNAR−/−, and TLR3−/− mice. Twelve-month-old B6 (A and B), 12- to 14-month-old IFNAR−/− (C and D), or 12- to 14-month-old TLR3−/− mice (E and F) were treated with IFN-β (2,000 U), IL-1β (50 ng), TNF (200 ng), IFN-γ (200 ng), IL-6 (50 ng), IL-12 (20 ng), GM-CSF (20 ng), or poly(I·C) (20 μg) 6 h before intranasal infection with 1 × 105 PFU MA15 virus. Weight loss and mortality were monitored daily. For B6 mice, n = 9 in the PBS group, 6 in the IFN-γ group, 6 in the IFN-β group, 10 in the IL-1β group, 8 in the IL-6 group, 10 in the IL-12 group, 6 in the TNF group, and 6 in the GM-CSF group. For IFNAR−/− mice, n = 6 in the PBS group, 6 in the poly(I·C) group, and 6 in the IFN-γ group. For TLR3−/− mice, n = 6 in the PBS group and 7 in the poly(I·C) group.
Fig 7
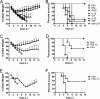
Poly(I·C) or cytokine treatments protect 22-month-old mice from lethal IAV infection. (A) Twenty-two-month-old B6 mice were treated with 20 μg poly(I·C), IFN-β (2,000 U), or IFN-γ (200 ng) 6 h or 6 and 48 h before infection with IAV. Weight loss and mortality were monitored daily. n = 14 mice in the PBS group, 7 in the −6-h poly(I·C) group, 8 in the −48-h/−6-h poly(I·C) group, 9 in the IFN-γ group, and 6 in the IFN-β group. (B) Twenty-two-month-old B6 mice were treated with poly(I·C) 48 and 6 h prior to intranasal infection with 1,066 TCID50 of IAV. RNA was extracted from lungs at the indicated time points and processed as described in Materials and Methods. n = 4 mice/group/experiment. Data are representative of 2 independent experiments. *, P < 0.05. (C) For virus titers, lungs were homogenized and titers of virus were determined on MDCK cells. Viral titers are expressed as TCID50/ml homogenate. n = 4 mice/group/time point. *, P < 0.05. Data are representative of 2 independent experiments.
Fig 8
Earlier inflammatory cell infiltration and decreased pulmonary edema occurs in poly(I·C)-treated IAV-infected mice. Twenty-two-month-old B6 mice were treated with 20 μg poly(I·C) or PBS 48 and 6 h before intranasal infection with IAV. (A) Lungs were removed at the indicated time points p.i., fixed in zinc formalin, and embedded in paraffin. Sections were stained with hematoxylin and eosin. Asterisks indicate edema; arrows indicate necrotic debris. Two mice were analyzed at each time point in each group. (B) Lungs were harvested at the indicated time points, and after enzyme digestion, single-cell suspensions were acquired. Total numbers of lung cells, rDCs (CD11c+ MHCII+ SiglecF−), alveolar macrophages (CD11c+ MHCII+/− SiglecF+), eosinophils (CD45+ CD11c+/− SiglecF+), and neutrophils (CD11b+ Ly6C+ Ly6G+) in the lung are shown. n = 3 mice/group/time point. *, P < 0.05.
Fig 9
Poly(I·C) is protective when delivered at low doses either several days prior to infection or systemically. Twelve-month-old B6 mice were treated with various doses of poly(I·C) 6 h before infection (termed the −6-h group) with 1 × 105 PFU MA15 (A), were treated with 20 μg poly(I·C) in 50 μl PBS intranasally (the 20-μg group) at different time points before or after infection (B), or were treated with 80 μg poly(I·C) in 200 μl PBS i.p., i.v., or s.c. 6 h before infection (C). (D) Twenty-two-month-old mice were treated with 80 μg poly(I·C) in 200 μl PBS i.p., i.v., or s.c. 6 h before IAV infection. Weight loss and mortality were monitored daily. For panel A, n = 8 mice in the PBS group, 13 in the 20-μg group, 8 in the 4-μg group, and 8 in the 0.8-μg group. For panel B, n = 9 mice in the PBS group, 6 in the −10-day group, 6 in the −5-day group, 10 in the −3-day group, 8 in the −6-h group, 10 in the +6-h group, and 6 in the +18-h group. For panel C, n = 6 mice in the PBS group, 6 in the i.v. group, 6 in the i.p. group, and 8 in the s.c. group. For panel D, n = 7 mice in the PBS group, 6 in the i.v. group, 8 in the i.p. group, and 6 in the s.c. group.
Similar articles
- Prophylactic and therapeutic intranasal administration with an immunomodulator, Hiltonol® (Poly IC:LC), in a lethal SARS-CoV-infected BALB/c mouse model.
Kumaki Y, Salazar AM, Wandersee MK, Barnard DL. Kumaki Y, et al. Antiviral Res. 2017 Mar;139:1-12. doi: 10.1016/j.antiviral.2016.12.007. Epub 2016 Dec 9. Antiviral Res. 2017. PMID: 27956136 Free PMC article. - Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection.
Channappanavar R, Fett C, Zhao J, Meyerholz DK, Perlman S. Channappanavar R, et al. J Virol. 2014 Oct;88(19):11034-44. doi: 10.1128/JVI.01505-14. Epub 2014 Jul 23. J Virol. 2014. PMID: 25056892 Free PMC article. - Complete protection against severe acute respiratory syndrome coronavirus-mediated lethal respiratory disease in aged mice by immunization with a mouse-adapted virus lacking E protein.
Fett C, DeDiego ML, Regla-Nava JA, Enjuanes L, Perlman S. Fett C, et al. J Virol. 2013 Jun;87(12):6551-9. doi: 10.1128/JVI.00087-13. Epub 2013 Apr 10. J Virol. 2013. PMID: 23576515 Free PMC article. - Induction of interferon-gamma-inducible protein 10 by SARS-CoV infection, interferon alfacon 1 and interferon inducer in human bronchial epithelial Calu-3 cells and BALB/c mice.
Kumaki Y, Day CW, Bailey KW, Wandersee MK, Wong MH, Madsen JR, Madsen JS, Nelson NM, Hoopes JD, Woolcott JD, McLean TZ, Blatt LM, Salazar AM, Smee DF, Barnard DL. Kumaki Y, et al. Antivir Chem Chemother. 2010 Mar 9;20(4):169-77. doi: 10.3851/IMP1477. Antivir Chem Chemother. 2010. PMID: 20231782 - Animal models in virus research: their utility and limitations.
Louz D, Bergmans HE, Loos BP, Hoeben RC. Louz D, et al. Crit Rev Microbiol. 2013 Nov;39(4):325-61. doi: 10.3109/1040841X.2012.711740. Epub 2012 Sep 14. Crit Rev Microbiol. 2013. PMID: 22978742 Review.
Cited by
- Prior Influenza Infection Mitigates SARS-CoV-2 Disease in Syrian Hamsters.
Di Pietro C, Haberman AM, Lindenbach BD, Smith PC, Bruscia EM, Allore HG, Vander Wyk B, Tyagi A, Zeiss CJ. Di Pietro C, et al. Viruses. 2024 Feb 3;16(2):246. doi: 10.3390/v16020246. Viruses. 2024. PMID: 38400021 Free PMC article. - Action Mechanisms and Scientific Rationale of Using Nasal Vaccine (HeberNasvac) for the Treatment of Chronic Hepatitis B.
Aguilar JC, Aguiar JA, Akbar SMF. Aguilar JC, et al. Vaccines (Basel). 2022 Dec 7;10(12):2087. doi: 10.3390/vaccines10122087. Vaccines (Basel). 2022. PMID: 36560498 Free PMC article. Review. - Exploiting viral sensing mediated by Toll-like receptors to design innovative vaccines.
Sartorius R, Trovato M, Manco R, D'Apice L, De Berardinis P. Sartorius R, et al. NPJ Vaccines. 2021 Oct 28;6(1):127. doi: 10.1038/s41541-021-00391-8. NPJ Vaccines. 2021. PMID: 34711839 Free PMC article. Review. - Immunological status of the olfactory bulb in a murine model of Toll-like receptor 3-mediated upper respiratory tract inflammation.
Kagoya R, Toma-Hirano M, Yamagishi J, Matsumoto N, Kondo K, Ito K. Kagoya R, et al. J Neuroinflammation. 2022 Jan 10;19(1):13. doi: 10.1186/s12974-022-02378-1. J Neuroinflammation. 2022. PMID: 35012562 Free PMC article. - Phagocytic cells contribute to the antibody-mediated elimination of pulmonary-infected SARS coronavirus.
Yasui F, Kohara M, Kitabatake M, Nishiwaki T, Fujii H, Tateno C, Yoneda M, Morita K, Matsushima K, Koyasu S, Kai C. Yasui F, et al. Virology. 2014 Apr;454-455:157-68. doi: 10.1016/j.virol.2014.02.005. Epub 2014 Mar 4. Virology. 2014. PMID: 24725942 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- P0106099/PHS HHS/United States
- R01 NS041249/NS/NINDS NIH HHS/United States
- R01 AI091322/AI/NIAID NIH HHS/United States
- P01 AI060699/AI/NIAID NIH HHS/United States
- R01AI091322/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous