Immune senescence: relative contributions of age and cytomegalovirus infection - PubMed (original) (raw)
Immune senescence: relative contributions of age and cytomegalovirus infection
Andrea Mekker et al. PLoS Pathog. 2012.
Abstract
Immune senescence, defined as the age-associated dysregulation and dysfunction of the immune system, is characterised by impaired protective immunity and decreased efficacy of vaccines. Recent clinical, epidemiological and immunological studies suggest that Cytomegalovirus (CMV) infection may be associated with accelerated immune senescence, possibly by restricting the naïve T cell repertoire. However, direct evidence whether and how CMV-infection is implicated in immune senescence is still lacking. In this study, we have investigated whether latent mouse CMV (MCMV) infection with or without thymectomy (Tx) alters antiviral immunity of young and aged mice. After infection with lymphocytic choriomeningitis virus (LCMV) or Vaccinia virus, specific antiviral T cell responses were significantly reduced in old, old MCMV-infected and/or Tx mice compared to young mice. Importantly, control of LCMV replication was more profoundly impaired in aged MCMV-infected mice compared to age-matched MCMV-naïve or young mice. In addition, latent MCMV infection was associated with slightly reduced vaccination efficacy in old Tx mice. In contrast to the prevailing hypothesis of a CMV-mediated restriction of the naïve T cell repertoire, we found similar naïve T cell numbers in MCMV-infected and non-infected mice, whereas ageing and Tx clearly reduced the naïve T cell pool. Instead, MCMV-infection expanded the total CD8(+) T cell pool by a massive accumulation of effector memory T cells. Based on these results, we propose a new model of increased competition between CMV-specific memory T cells and any 'de novo' immune response in aged individuals. In summary, our results directly demonstrate in a mouse model that latent CMV-infection impairs immunity in old age and propagates immune senescence.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
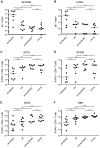
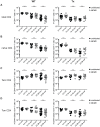
Figure 1. LCMV-clearance and LCMV- or VACV-specific CD8+ T cell responses in young and old mice with/without latent MCMV-infection.
A–D: uninfected or MCMV infected (107 pfu MCMV-Δ157 i.v.) young (4 or 6 months old; 2 or 4 months p.i.) and old (15 or 22 months old; 11 or 18 months p.i.) C57BL/6 mice were infected with 2×103 pfu LCMV-WE i.v. Eight days after infection, LCMV titres were determined in spleen (A) and lung (B) by plaque forming assay and the virus-specific CD8+ T cell response was quantified by ICS in the lung for GP33-specific (C) and NP396-specific (D) CD8+ T cells. E, F: young (4 months old, 2 months p.i.) and old C57BL/6 mice (25 or 28 months old, 23 or 26 months p.i.) with and without latent MCMV-infection were infected with 5×106 pfu VACV-GP i.p. At the peak of the response on day 6, GP33-specific (E) and B8R-specific (F) CD8+ T cells from the lung were quantified by ICS after in vitro re-stimulation with the respective peptide. Circles indicate total numbers of epitope specific CD8+ T cells of individual mice, horizontal lines show the medians of an individual group. Data are pooled from two independent experiments. ANOVA followed by Bonferroni post-analysis was used to determine significant differences between the displayed medians of each group (* p<0.05; ** p<0.01; ns = not significant). The dotted line corresponds to the detection limit of the assay. p.i., post infection.
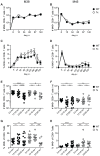
Figure 2. Long-term analysis of MCMV-specific CD8+ T cell responses in thymectomised and non-thymectomised mice.
C57BL/6 mice were thymectomised at age of 4–5 weeks. Two to 3 weeks later, Tx mice (open circles) and non-Tx wild type mice (wt, closed circles) were infected with 107 pfu MCMV-Δ157 i.v. The MCMV-specific CD8+ T cells were quantified in the blood by tetramer staining and analysed by polychromatic flow cytometry using beads for direct measurements of total numbers. A–D: longitudinal analysis of the total number of M38- (A) and M45-specific (B) CD8+ T cells in the blood. Longitudinal analysis of the frequencies of M38- (C) and M45-specific (D) CD8+ T cells in the blood at different time points after MCMV infection. Circles indicate the mean of 8–11 (A, B) or 5–7 (C, D) mice per group, error bars indicate the SEM. Figure E–H: cross-sectional analysis of the total numbers (E, F) and the frequencies (G, H) of M38- (E, G) and M45-specific (F, H) CD8+ T cells in the blood of young (3–4 months), middle-aged (13–18 months) and old C57BL/6 mice (23–28 months). Data of the cross-sectional analysis were pooled from three independent experiments. ANOVA followed by Bonferroni post-analysis was carried out for calculating significance (* p<0.05; ** p<0.01; *** p<0.001; ns = not significant). p.i., post infection.
Figure 3. Immunogenicity and efficacy of VLP-immunisation against LCMV-challenge in young and old mice with/without Tx or latent MCMV-infection.
Young (5 months old, 3 months p.i.) and old C57BL/6 mice (18 months old, 15 months p.i.) with or without Tx and latent MCMV-infection were immunised with 10 µg VLP-GP33 s.c. Thereafter, the GP33-specific CD8+ T cell response was measured on day 7 (A) by tetramer staining and the Qb-specific IgG titres were quantified by ELISA on day 10 (E) and day 20 (F) in the blood. On day 21, all mice were challenged with 2×103 pfu LCMV-WE i.v. Eight days after LCMV-challenge the GP33-specific CD8+ T cell response was determined by ICS in the lung (B) and LCMV-titres were determined in the spleen (C) and in the lung (D) by plaque assay. Circles indicate individual mice, horizontal lines represent the median (A–D). Vertical bars show the mean of an experimental group, error bars correspond to the SEM (E, F). The dotted line shows the detection limit of the assay. 4–5 mice per group were included. ANOVA followed by Bonferroni post-analysis was preformed to test for significant differences (* p<0.05; ** p<0.01; ns = not significant).
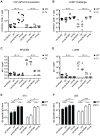
Figure 4. Analysis of virus specific T cell responses after adoptive transfer of TCR transgenic CD8+ and CD4+ T cells and VACV-GP infection in young and old mice with/without Tx or latent MCMV-infection.
Transgenic GP33-specific CD8+ and GP61-specific CD4+ T cells were isolated from the spleen of young Ly5.1+ donor mice and transferred into young (4–6 months old) or old (23–28 months old), MCMV-infected (2–4 or 20–25 months p.i.) or uninfected, Tx (open circles) or non-Tx (wt, closed circles) recipients (Ly5.2). One day later, mice were infected with 5×106 pfu VACV-GP i.p.. Six days later, VACV-GP-specific CD4+ and CD8+ T cell responses were measured in the lung by tetramer-staining (A) or by ICS after in vitro re-stimulation with the respective peptide (B–D) and analysed by flow cytrometry. (A) Total number of GP33-specific transgenic CD8+ T cells (Ly5.1) in the lung. (B) Total number of GP61-specific transgenic CD4+ T cells (Ly5.1) in the lung. (C) Total number of endogenous GP33-specific CD8+ T cells (Ly5.2) in the lung. (D) Total number of endogenous A8R-specific CD8+ T cells in the lung. Pooled data from two independent experiments are displayed. Circles indicate values of individual mice, horizontal bars indicate the medians. Significance was assessed by ANOVA followed by Bonferroni post-analysis (* p<0.05; ** p<0.01; ns = not significant).
Figure 5. Long-term analysis of the CD8+ T cell compartment after MCMV infection and/or thymectomy in young, middle-aged and old mice.
C57BL/6 mice were thymectomised at age of 4–5 weeks. Two to 3 weeks later, Tx (right panel) and non-Tx wild type (WT, left panel) mice were infected with 107 pfu MCMV-Δ157 i.v. The absolute number of CD8+ T cells was quantified in the blood and phenotypically characterised by polychromatic flow cytometry using CD44 and CD62L. Quantitative analysis of total CD8+ T cell numbers (A), naïve CD8+ T cell numbers (B; CD8+CD44−CD62L+), effector memory (Tem) CD8+ T cell numbers (C; CD8+CD44+CD62L−) and central memory (Tcm) CD8+ T cell numbers (D; CD8+CD44+CD62L+). Data were pooled from three independent experiments. Circles indicate values of individual mice, horizontal bars correspond to the median of an experimental group. Significance was assessed by ANOVA followed by Bonferroni post-analysis (* p<0.05; ** p<0.01; *** p<0.001; ns = not significant).
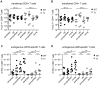
Figure 6. Alterations of CD4+ and CD8+ T cell populations in secondary lymphoid and non-lymphoid organs of middle-aged mice with (open symbols) and without (closed symbols) latent MCMV-infection.
Half of the mice were infected with 107 pfu MCMV-Δ157 i.v. at the age of 8 weeks. At the age of 8 (triangles) or 13 (circles) months, blood was taken, mice were perfused and sacrificed and total numbers of naïve (CD44−CD62L+) CD8+ (A) and CD4+ (D) T cells, effector memory (Tem; CD44+CD62L−) CD8+ (B) and CD4+ (E) T cells and central memory (Tcm; CD44+CD62L+) CD8+ (C) and CD4+ (F) T cells were determined in different organs by polychromatic flow cytometry using bead calibration. Absolute numbers of T cells are given for blood, lung, spleen, cervical (cer) and inguinal (in) lymph nodes (LN). Circles indicate values of individual mice, horizontal bars correspond to the median of an experimental group. Significance was assessed by t-test (* p<0.05; ** p<0.01; *** p<0.001; ns = not significant).
Figure 7. Long-term analysis of the CD4+ T cell compartment after MCMV infection and/or thymectomy in young, middle-aged and old mice.
C57BL/6 mice were thymectomised at age of 4–5 weeks. Two to 3 weeks later, Tx (right panel) and non-Tx wild type (WT, left panel) mice were infected with 107 pfu MCMV-Δ157 i.v. The absolute number of CD4+ T cells was quantified in the blood and phenotypically characterised by polychromatic flow cytometry using CD44 and CD62L. Quantitative analysis of total CD4+ T cell numbers (A), naïve CD8+ T cell numbers (B; CD4+CD44−CD62L+), effector memory (Tem) CD4+ T cell numbers (C; CD4+CD44+CD62L−) and central memory (Tcm) CD4+ T cell numbers (D; CD4+CD44+CD62L+). Data were pooled from three independent experiments. Circles indicate values of individual mice, horizontal bars correspond to the median of an experimental group. Significance was assessed by ANOVA followed by Bonferroni post-analysis (* p<0.05; ns = not significant).
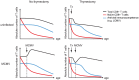
Figure 8. Integrative model of the influence of MCMV-infection, ageing and thymectomy on CD8+ T cell populations and protective immunity.
Ageing is accompanied by a slow but progressive decline of the total (black line) and the naïve (red line) CD8+ T cell pool. Immune senescence is characterised by an age-associated reduction of immunocompetence (blue line), particularly against newly encountered antigens (e.g. LCMV). Tx of young mice leads to a substantial and rapid shrinking of the naïve T cell compartment reducing the number and the diversity of naïve T cell precursors on top of ageing. Tx also reduces the total CD8+ T compartment, which is later affected by an age-associated decline. MCMV-infection results in an immediate and persistent expansion of the total CD8+ T cell compartment whereas the naïve T cell pool remains intact. Nevertheless, MCMV-infection contributes to reduced immunocompetence over time, possibly by an enhanced competition of massively expanded CMV-specific Tem with newly generated effector cells. In old Tx mice with latent MCMV-infection, immuocompetence is cumulatively affected by the restricted naïve T cell pool and by the increased competition of CMV-specific Tem.
Similar articles
- Cytomegalovirus infection impairs immune responses and accentuates T-cell pool changes observed in mice with aging.
Cicin-Sain L, Brien JD, Uhrlaub JL, Drabig A, Marandu TF, Nikolich-Zugich J. Cicin-Sain L, et al. PLoS Pathog. 2012;8(8):e1002849. doi: 10.1371/journal.ppat.1002849. Epub 2012 Aug 16. PLoS Pathog. 2012. PMID: 22916012 Free PMC article. - Heterologous Immunity and Persistent Murine Cytomegalovirus Infection.
Che JW, Daniels KA, Selin LK, Welsh RM. Che JW, et al. J Virol. 2017 Jan 3;91(2):e01386-16. doi: 10.1128/JVI.01386-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27807227 Free PMC article. - Antiviral therapy can reverse the development of immune senescence in elderly mice with latent cytomegalovirus infection.
Beswick M, Pachnio A, Lauder SN, Sweet C, Moss PA. Beswick M, et al. J Virol. 2013 Jan;87(2):779-89. doi: 10.1128/JVI.02427-12. Epub 2012 Oct 31. J Virol. 2013. PMID: 23115277 Free PMC article. - Cytomegalovirus immune evasion sets the functional avidity threshold for protection by CD8 T cells.
Hamdan S, Reddehase MJ, Holtappels R. Hamdan S, et al. Med Microbiol Immunol. 2023 Apr;212(2):153-163. doi: 10.1007/s00430-022-00733-w. Epub 2022 Apr 1. Med Microbiol Immunol. 2023. PMID: 35364731 Free PMC article. Review. - NK cells controlling virus-specific T cells: Rheostats for acute vs. persistent infections.
Welsh RM, Waggoner SN. Welsh RM, et al. Virology. 2013 Jan 5;435(1):37-45. doi: 10.1016/j.virol.2012.10.005. Virology. 2013. PMID: 23217614 Free PMC article. Review.
Cited by
- Late-life Attenuation of Cytomegalovirus-mediated CD8 T Cell Memory Inflation: Shrinking of the Cytomegalovirus Latency Niche.
Coplen CP, Sonar SA, Nikolich JŽ. Coplen CP, et al. J Immunol. 2024 Oct 1;213(7):965-970. doi: 10.4049/jimmunol.2400113. J Immunol. 2024. PMID: 39150241 - Immunosenescence and Inflammation in Chronic Obstructive Pulmonary Disease: A Systematic Review.
Ramos Jesus F, Correia Passos F, Miranda Lopes Falcão M, Vincenzo Sarno Filho M, Neves da Silva IL, Santiago Moraes AC, Lima Costa Neves MC, Baccan GC. Ramos Jesus F, et al. J Clin Med. 2024 Jun 13;13(12):3449. doi: 10.3390/jcm13123449. J Clin Med. 2024. PMID: 38929978 Free PMC article. Review. - Single-cell profiling of immune system alterations in lymphoid, barrier and solid tissues in aged mice.
Krishnarajah S, Ingelfinger F, Friebel E, Cansever D, Amorim A, Andreadou M, Bamert D, Litscher G, Lutz M, Mayoux M, Mundt S, Ridder F, Sparano C, Stifter SA, Ulutekin C, Unger S, Vermeer M, Zwicky P, Greter M, Tugues S, De Feo D, Becher B. Krishnarajah S, et al. Nat Aging. 2022 Jan;2(1):74-89. doi: 10.1038/s43587-021-00148-x. Epub 2021 Dec 16. Nat Aging. 2022. PMID: 37118354 - Interactomics: Dozens of Viruses, Co-evolving With Humans, Including the Influenza A Virus, may Actively Distort Human Aging.
Teulière J, Bernard C, Bonnefous H, Martens J, Lopez P, Bapteste E. Teulière J, et al. Mol Biol Evol. 2023 Feb 3;40(2):msad012. doi: 10.1093/molbev/msad012. Mol Biol Evol. 2023. PMID: 36649176 Free PMC article. - Immunosenescence and inflammaging in the aged horse.
DeNotta S, McFarlane D. DeNotta S, et al. Immun Ageing. 2023 Jan 6;20(1):2. doi: 10.1186/s12979-022-00325-5. Immun Ageing. 2023. PMID: 36609345 Free PMC article. Review.
References
- Castle SC (2000) Clinical relevance of age-related immune dysfunction. Clin Infect Dis 31: 578–585. - PubMed
- Hainz U, Jenewein B, Asch E, Pfeiffer KP, Berger P, et al. (2005) Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine 23: 3232–3235. - PubMed
- Musher DM, Chapman AJ, Goree A, Jonsson S, Briles D, et al. (1986) Natural and vaccine-related immunity to Streptococcus pneumoniae. J Infect Dis 154: 245–256. - PubMed
- Messaoudi I, Warner J, Nikolich-Zugich D, Fischer M, Nikolich-Zugich J (2006) Molecular, cellular, and antigen requirements for development of age-associated T cell clonal expansions in vivo. J Immunol 176: 301–308. - PubMed
- Messaoudi I, Warner J, Nikolich-Zugich J (2006) Age-related CD8+ T cell clonal expansions express elevated levels of CD122 and CD127 and display defects in perceiving homeostatic signals. J Immunol 177: 2784–2792. - PubMed
Publication types
MeSH terms
Grants and funding
This work was supported by the Swiss National Science Foundation (grant PP0033-110737 to UK, www.snf.ch), the Aetas Foundation (Geneva, Switzerland, www.aetas.ch) and from the Heuberg Foundation (Zürich, Switzerland). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials