Native tandem and ion mobility mass spectrometry highlight structural and modular similarities in clustered-regularly-interspaced shot-palindromic-repeats (CRISPR)-associated protein complexes from Escherichia coli and Pseudomonas aeruginosa - PubMed (original) (raw)
Native tandem and ion mobility mass spectrometry highlight structural and modular similarities in clustered-regularly-interspaced shot-palindromic-repeats (CRISPR)-associated protein complexes from Escherichia coli and Pseudomonas aeruginosa
Esther van Duijn et al. Mol Cell Proteomics. 2012 Nov.
Abstract
The CRISPR/Cas (clustered regularly interspaced short palindromic repeats/CRISPR-associated genes) immune system of bacteria and archaea provides acquired resistance against viruses and plasmids, by a strategy analogous to RNA-interference. Key components of the defense system are ribonucleoprotein complexes, the composition of which appears highly variable in different CRISPR/Cas subtypes. Previous studies combined mass spectrometry, electron microscopy, and small angle x-ray scattering to demonstrate that the E. coli Cascade complex (405 kDa) and the P. aeruginosa Csy-complex (350 kDa) are similar in that they share a central spiral-shaped hexameric structure, flanked by associating proteins and one CRISPR RNA. Recently, a cryo-electron microscopy structure of Cascade revealed that the CRISPR RNA molecule resides in a groove of the hexameric backbone. For both complexes we here describe the use of native mass spectrometry in combination with ion mobility mass spectrometry to assign a stable core surrounded by more loosely associated modules. Via computational modeling subcomplex structures were proposed that relate to the experimental IMMS data. Despite the absence of obvious sequence homology between several subunits, detailed analysis of sub-complexes strongly suggests analogy between subunits of the two complexes. Probing the specific association of E. coli Cascade/crRNA to its complementary DNA target reveals a conformational change. All together these findings provide relevant new information about the potential assembly process of the two CRISPR-associated complexes.
Figures
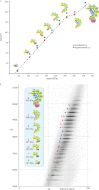
Fig. 1.
A, Native mass spectrum of intact Cascade, showing a dominant charge state distribution that is consistent with a species of 405,365 ± 135 Da. Two additional subcomplexes were identified having molecular masses of 349,399 ± 84 Da (Cascade lacking Cse1) and 42,524 ± 8 Da (dimeric Cse2, inset). B, Mass spectrum of Cascade sprayed from a solution containing 5% iso-propanol, triggering in solution dissociation of the complex. Several additional charge state distributions that correspond to Cascade subcomplexes were identified. All complexes annotated in (A) and (B) are indicated by colors shown in the inset on the right. C, Native mass spectrum of the Csy system of Pseudomonas aeruginosa, showing a dominant charge state distribution that is consistent with a the intact complex of 350,414 ± 22 Da. Satellite peaks originate from incomplete removal of the purification tag of Csy4, one of the complex components, from the complex resulting into a complex mass of 352,494 ± 16. In addition two subcomplexes were identified having molecular masses of 265,307 ± 9 Da, lacking both Csy1 and Csy2, and a dimeric subcomplex of the endoribonuclease Csy4 and crRNA (40.867 Da), see inset. Adapted from (22).
Fig. 2.
Subunit connectivity diagram of Cascade, indicating a stable core composed of Cas71Cas51Cas6e1/crRNA1. For each protein-protein or protein-crRNA interaction a connectivity score is given, which is based on the observed frequency of that specific contact. Two loosely associated modules were identified, Cse1 and dimeric Cse2, both have low scores.
Fig. 3.
A, Plot of the Ω versus the mass of Cascade sub(complexes). The experimental values are plotted in orange, the theoretical Ω 's calculated from the modeled structures are depicted in purple. A near linear trend line is observed upon the dissociation of each Cas7 subunit from the complex, suggesting the formation of an open string-like scaffold by the six Cas7 proteins. The linear correlation between Ω and mass is not valid when dimeric Cse2 disassembles from the complex, likely because it occupies a cavity of the complex (boxed). The release Cse1 considerably decreases the Ω of Cascade, confirming the peripheral location of this subunit within Cascade. B, Drift time chromatogram, showing the separation of Cascade and the subcomplexes, based on drift time (shown in bins, 100 bins equals 18 ms) and m/z. Subcomplexes are color-coded. The inset identifies the various species.
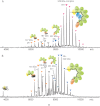
Fig. 4.
A, Native mass spectrum of Cse22Cas76Cas51Cas6e1/crRNA1. The dominant species present corresponds to a mass of 349,407 ± 43 Da, in good agreement with the expected stoichiometry (theoretical mass 349,051 Da). B, Native mass spectrum of Cas76Cas51Cas6e1/crRNA1, with a mass of 324,546 ± 96 Da. The expression of the complex without the presence of Cse1 and Cse2 destabilizes Cascade, as shown by the increased abundance of several subcomplexes. All subcomplexes were previously observed after the in solution dissociation of complete Cascade by adding 5% iso-propanol.
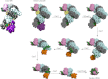
Fig. 5.
Modeled structures for Cascade subcomplexes based on the cryo-EM density map (20). A dummy-atom filled model was generated from the EM data of the intact complex. Models of the smaller modules were based on the structure of the intact complex, lacking specific subunits, whereby the crRNA (in green) was allowed to rearrange to contact tightly on to the remaining proteins. The other Cas subunits are depicted in the following colors: Cse1, purple; Cse2, yellow; Cas7, gray and blue (for clarity the consecutive Cas7 subunits alternate in color); Cas5, orange; and Cas6e, pink.
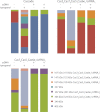
Fig. 6.
Bar diagrams summarizing the summed intensities of all complexes present for (A) Cascade, (B) Cse22Cas76Cas51Cas6e1/crRNA1, and (C) Cas76Cas51Cas6e1/crRNA1 under varying conditions (with and without i-propanol or ssDNA). The overall conclusion from these experiments is that ssDNA binding significantly increases the stability of Cascade, Cse22Cas76Cas51Cas6e1/crRNA1 or Cas76Cas51Cas6e1/crRNA1. Clearly a reduction in intermediate complexes formed is observed, after triggering in solution dissociation of the complexes by the addition of iso-propanol to the electrospray solution.
Similar articles
- Crystal structure of the RNA-guided immune surveillance Cascade complex in Escherichia coli.
Zhao H, Sheng G, Wang J, Wang M, Bunkoczi G, Gong W, Wei Z, Wang Y. Zhao H, et al. Nature. 2014 Nov 6;515(7525):147-50. doi: 10.1038/nature13733. Epub 2014 Aug 12. Nature. 2014. PMID: 25118175 - Formation and Stoichiometry of CRISPR-Cascade Complexes with Varying Spacer Lengths Revealed by Native Mass Spectrometry.
Wittig S, Songailiene I, Schmidt C. Wittig S, et al. J Am Soc Mass Spectrom. 2020 Mar 4;31(3):538-546. doi: 10.1021/jasms.9b00011. Epub 2020 Jan 30. J Am Soc Mass Spectrom. 2020. PMID: 32008319 - Modulating the Cascade architecture of a minimal Type I-F CRISPR-Cas system.
Gleditzsch D, Müller-Esparza H, Pausch P, Sharma K, Dwarakanath S, Urlaub H, Bange G, Randau L. Gleditzsch D, et al. Nucleic Acids Res. 2016 Jul 8;44(12):5872-82. doi: 10.1093/nar/gkw469. Epub 2016 May 23. Nucleic Acids Res. 2016. PMID: 27216815 Free PMC article. - Clustered regularly interspaced short palindromic repeats (CRISPRs): the hallmark of an ingenious antiviral defense mechanism in prokaryotes.
Al-Attar S, Westra ER, van der Oost J, Brouns SJ. Al-Attar S, et al. Biol Chem. 2011 Apr;392(4):277-89. doi: 10.1515/BC.2011.042. Epub 2011 Feb 7. Biol Chem. 2011. PMID: 21294681 Review. - Function and regulation of clustered regularly interspaced short palindromic repeats (CRISPR) / CRISPR associated (Cas) systems.
Richter C, Chang JT, Fineran PC. Richter C, et al. Viruses. 2012 Oct 19;4(10):2291-311. doi: 10.3390/v4102291. Viruses. 2012. PMID: 23202464 Free PMC article. Review.
Cited by
- CRISPR-Cas9 Genome Editing in Human Cell Lines with Donor Vector Made by Gibson Assembly.
Sahoo N, Cuello V, Udawant S, Litif C, Mustard JA, Keniry M. Sahoo N, et al. Methods Mol Biol. 2020;2115:365-383. doi: 10.1007/978-1-0716-0290-4_20. Methods Mol Biol. 2020. PMID: 32006411 Free PMC article. - CRISPR-Cas: evolution of an RNA-based adaptive immunity system in prokaryotes.
Koonin EV, Makarova KS. Koonin EV, et al. RNA Biol. 2013 May;10(5):679-86. doi: 10.4161/rna.24022. Epub 2013 Feb 25. RNA Biol. 2013. PMID: 23439366 Free PMC article. Review. - Comparative genomics of defense systems in archaea and bacteria.
Makarova KS, Wolf YI, Koonin EV. Makarova KS, et al. Nucleic Acids Res. 2013 Apr;41(8):4360-77. doi: 10.1093/nar/gkt157. Epub 2013 Mar 6. Nucleic Acids Res. 2013. PMID: 23470997 Free PMC article. Review. - Dynamic protein ligand interactions--insights from MS.
Schmidt C, Robinson CV. Schmidt C, et al. FEBS J. 2014 Apr;281(8):1950-64. doi: 10.1111/febs.12707. Epub 2014 Jan 21. FEBS J. 2014. PMID: 24393119 Free PMC article. Review. - Structural analyses of the CRISPR protein Csc2 reveal the RNA-binding interface of the type I-D Cas7 family.
Hrle A, Maier LK, Sharma K, Ebert J, Basquin C, Urlaub H, Marchfelder A, Conti E. Hrle A, et al. RNA Biol. 2014;11(8):1072-82. doi: 10.4161/rna.29893. RNA Biol. 2014. PMID: 25483036 Free PMC article.
References
- Horvath P., Barrangou R. (2010) CRISPR/Cas, the immune system of bacteria and archaea. Science 327, 167–170 - PubMed
- Makarova K. S., Grishin N. V., Shabalina S. A., Wolf Y. I., Koonin E. V. (2006) A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol. Direct 1, 7. - PMC - PubMed
- Van der Oost J., Jore M. M., Westra E. R., Lundgren M., Brouns S. J. (2009) CRISPR-based adaptive and heritable immunity in prokaryotes. Trends Biochem. Sci. 34, 401–407 - PubMed
- Deveau H., Garneau J. E., Moineau S. (2010) CRISPR/Cas system and its role in phage-bacteria interactions. Ann. Rev. Microbiol. 64, 475–493 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases