Coffee polyphenols change the expression of STAT5B and ATF-2 modifying cyclin D1 levels in cancer cells - PubMed (original) (raw)
Coffee polyphenols change the expression of STAT5B and ATF-2 modifying cyclin D1 levels in cancer cells
Carlota Oleaga et al. Oxid Med Cell Longev. 2012.
Abstract
Background: Epidemiological studies suggest that coffee consumption reduces the risk of cancer, but the molecular mechanisms of its chemopreventive effects remain unknown.
Objective: To identify differentially expressed genes upon incubation of HT29 colon cancer cells with instant caffeinated coffee (ICC) or caffeic acid (CA) using whole-genome microarrays.
Results: ICC incubation of HT29 cells caused the overexpression of 57 genes and the underexpression of 161, while CA incubation induced the overexpression of 12 genes and the underexpression of 32. Using Venn-Diagrams, we built a list of five overexpressed genes and twelve underexpressed genes in common between the two experimental conditions. This list was used to generate a biological association network in which STAT5B and ATF-2 appeared as highly interconnected nodes. STAT5B overexpression was confirmed at the mRNA and protein levels. For ATF-2, the changes in mRNA levels were confirmed for both ICC and CA, whereas the decrease in protein levels was only observed in CA-treated cells. The levels of cyclin D1, a target gene for both STAT5B and ATF-2, were downregulated by CA in colon cancer cells and by ICC and CA in breast cancer cells.
Conclusions: Coffee polyphenols are able to affect cyclin D1 expression in cancer cells through the modulation of STAT5B and ATF-2.
Figures
Figure 1
Biological association network (BAN) of differentially expressed genes in common between CA and ICC. The list of common genes between both treatments was used to construct a BAN with the Pathway Analysis software within GeneSpring v.11.5.1. An expanded network was constructed by setting an advanced filter that included the categories of binding, expression, metabolism, promoter binding, protein modification, and regulation. Only proteins are represented. The BAN shows the node genes STAT5B and ATF-2 that were further studied.
Figure 2
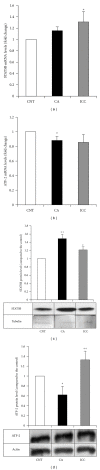
Quantitation of mRNA and protein levels for STAT5B and ATF-2 in HT29 cells. The mRNA levels of STAT5B (a) and ATF-2 (b) were determined in control HT29 cells (empty bars) and cells treated with caffeic acid (CA, filled bars) and instant caffeinated coffee (ICC, grey bars) by RT real-time PCR as described in Methods. Results are expressed in fold changes compared to the control and are the mean ± SE of 3 different experiments. *P < 0.05 compared with the corresponding control. The protein levels of STAT5B (c) and ATF-2 (d) were determined in control HT29 cells (empty bars) and cells treated with caffeic acid (CA, filled bars) and instant caffeinated coffee (ICC, grey bars) by Western blot. Blots were reprobed with an antibody against _β_-actin or tubulin to normalize the results. Results represent the mean ± SE of 3 different experiments. *P < 0.05 and **P < 0.01 compared with the corresponding control.
Figure 3
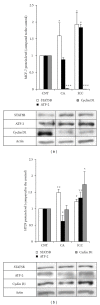
Expression of cyclin D1 upon incubation with ICC and CA in HT29 and MCF-7 cells. (a) Quantitation of STAT5b (empty bars), ATF-2 (filled bars), and cyclin D1 (grey bars) protein levels in MCF-7 cells. The protein levels were determined in control MCF-7 cells (CNT) and cells treated with caffeic acid (CA) and instant coffee (ICC) by Western blot. Blots were reprobed with an antibody against _β_-actin to normalize the results. Results represent the mean ± SE of 3 different experiments. *P < 0.05 and ***P < 0.001 compared with the corresponding control. (b) Quantitation of STAT5b (empty bars), ATF-2 (filled bars), and cyclin D1 (grey bars) protein levels in HT29 cells. The protein levels were determined in control HT29 cells (CNT) and cells treated with caffeic acid (CA) and instant coffee (ICC) by Western blot. Blots were reprobed with an antibody against _β_-actin to normalize the results. Results represent the mean ± SE of 3 different experiments.*P < 0.05 and **P < 0.01 compared with the corresponding control.
Similar articles
- Signal transducer and activator of transcription 5b, c-Src, and epidermal growth factor receptor signaling play integral roles in estrogen-stimulated proliferation of estrogen receptor-positive breast cancer cells.
Fox EM, Bernaciak TM, Wen J, Weaver AM, Shupnik MA, Silva CM. Fox EM, et al. Mol Endocrinol. 2008 Aug;22(8):1781-96. doi: 10.1210/me.2007-0419. Epub 2008 Jun 11. Mol Endocrinol. 2008. PMID: 18550772 Free PMC article. - Hemin inhibits cyclin D1 and IGF-1 expression via STAT5b under hypoxia in ERalpha-negative MDA-MB 231 breast cancer cells.
Lim EJ, Joung YH, Jung SM, Park SH, Park JH, Kim SY, Hwang TS, Hong DY, Chung SC, Ye SK, Moon ES, Park EU, Park T, Chung IM, Yang YM. Lim EJ, et al. Int J Oncol. 2010 May;36(5):1243-51. doi: 10.3892/ijo_00000608. Int J Oncol. 2010. PMID: 20372799 - Enhancement of hypoxia-induced apoptosis of human breast cancer cells via STAT5b by momilactone B.
Joung YH, Lim EJ, Kim MS, Lim SD, Yoon SY, Lim YC, Yoo YB, Ye SK, Park T, Chung IM, Bae KY, Yang YM. Joung YH, et al. Int J Oncol. 2008 Sep;33(3):477-84. Int J Oncol. 2008. PMID: 18695876 - METTL3-STAT5B interaction facilitates the co-transcriptional m6A modification of mRNA to promote breast tumorigenesis.
Bhattarai PY, Kim G, Lim SC, Choi HS. Bhattarai PY, et al. Cancer Lett. 2024 Oct 28;603:217215. doi: 10.1016/j.canlet.2024.217215. Epub 2024 Aug 31. Cancer Lett. 2024. PMID: 39218290 - Mechanism of cyclin D1 (CCND1, PRAD1) overexpression in human cancer cells: analysis of allele-specific expression.
Hosokawa Y, Arnold A. Hosokawa Y, et al. Genes Chromosomes Cancer. 1998 May;22(1):66-71. doi: 10.1002/(sici)1098-2264(199805)22:1<66::aid-gcc9>3.0.co;2-5. Genes Chromosomes Cancer. 1998. PMID: 9591636 Review.
Cited by
- Severe periodontitis is inversely associated with coffee consumption in the maintenance phase of periodontal treatment.
Machida T, Tomofuji T, Ekuni D, Azuma T, Takeuchi N, Maruyama T, Mizutani S, Kataoka K, Kawabata Y, Morita M. Machida T, et al. Nutrients. 2014 Oct 21;6(10):4476-90. doi: 10.3390/nu6104476. Nutrients. 2014. PMID: 25338270 Free PMC article. - Effects of Coffee and Its Components on the Gastrointestinal Tract and the Brain-Gut Axis.
Iriondo-DeHond A, Uranga JA, Del Castillo MD, Abalo R. Iriondo-DeHond A, et al. Nutrients. 2020 Dec 29;13(1):88. doi: 10.3390/nu13010088. Nutrients. 2020. PMID: 33383958 Free PMC article. Review. - Potential Health Benefits of Olive Oil and Plant Polyphenols.
Gorzynik-Debicka M, Przychodzen P, Cappello F, Kuban-Jankowska A, Marino Gammazza A, Knap N, Wozniak M, Gorska-Ponikowska M. Gorzynik-Debicka M, et al. Int J Mol Sci. 2018 Feb 28;19(3):686. doi: 10.3390/ijms19030686. Int J Mol Sci. 2018. PMID: 29495598 Free PMC article. Review. - The Impact of Coffee and Its Selected Bioactive Compounds on the Development and Progression of Colorectal Cancer In Vivo and In Vitro.
Bułdak RJ, Hejmo T, Osowski M, Bułdak Ł, Kukla M, Polaniak R, Birkner E. Bułdak RJ, et al. Molecules. 2018 Dec 13;23(12):3309. doi: 10.3390/molecules23123309. Molecules. 2018. PMID: 30551667 Free PMC article. Review. - ATF7 is stabilized during mitosis in a CDK1-dependent manner and contributes to cyclin D1 expression.
Schaeffer E, Vigneron M, Sibler AP, Oulad-Abdelghani M, Chatton B, Donzeau M. Schaeffer E, et al. Cell Cycle. 2015;14(16):2655-66. doi: 10.1080/15384101.2015.1064568. Cell Cycle. 2015. PMID: 26101806 Free PMC article.
References
- Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. Dietary polyphenols and the prevention of diseases. Critical Reviews in Food Science and Nutrition. 2005;45(4):287–306. - PubMed
- Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: antioxidants and beyond. The American Journal of Clinical Nutrition. 2005;81(1):215S–217S. - PubMed
- Azzi A, Davies KJ, Kelly F. Free radical biology—terminology and critical thinking. FEBS Letters. 2004;558(1–3):3–6. - PubMed
- Halliwell B, Rafter J, Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? The American Journal of Clinical Nutrition. 2005;81(1):268S–276S. - PubMed
- Olthof MR, Hollman PC, Katan MB. Chlorogenic acid and caffeic acid are absorbed in humans. Journal of Nutrition. 2001;131(1):66–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous