Flow control in our vessels: vascular valves make sure there is no way back - PubMed (original) (raw)
Review
Flow control in our vessels: vascular valves make sure there is no way back
Eleni Bazigou et al. Cell Mol Life Sci. 2013 Mar.
Abstract
The efficient transport of blood and lymph relies on competent intraluminal valves that ensure unidirectional fluid flow through the vessels. In the lymphatic vessels, lack of luminal valves causes reflux of lymph and can lead to lymphedema, while dysfunction of venous valves is associated with venous hypertension, varicose veins, and thrombosis that can lead to edema and ulcerations. Despite their clinical importance, the mechanisms that regulate valve formation are poorly understood and have only recently begun to be characterized. Here, we discuss new findings regarding the development of venous and lymphatic valves that indicate the involvement of common molecular mechanisms in regulating valve formation in different vascular beds.
Figures
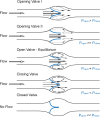
Fig. 1
Fluid dynamics of a valve. Distinct phases of opening and closing of valve leaflets (blue): opening, equilibrium, closed, and closing, modified from [12]. Fluid pressure drop across the vessel drives direction of flow (black hollow arrow), while forces on either side of valve leaflets (blue, P open, P close) determine the leaflets’ position inside the lumen and the size of the valve orifice. Black arrows point at the distinct flow patterns as well as the fluid velocities at different parts of the valve pocket (relatively scaled to demonstrate magnitude differences), such as axial flow in the middle of the vessel and detached streamlines at the free ends of the open leaflets developing into vortices in the sinus. Gray line shows the level of the vessel distension during the opening phase of the valve
Fig. 2
Similarities and differences between valve formation process in veins and lymphatic vessels. a First drawing of a valve, by Salomon Alberti in 1585 ([28], reproduced from [27], Copyright (1927), Royal Society Medicine Press, UK), showing the outside and inside of part of a leg vein (indicated by A, B) with a tributary vein (C). D and E indicate the two cusps of a bicuspid valve. b Transmission electron microscopy of the tip of a lymphatic valve leaflet L lumen, S sinus. Matrix core of the leaflet is highlighted in pink and flow direction is indicated by an arrow. Scale bar 2 μm. c Schematic of the developmental process of valve formation in veins (top row, blue) and lymphatic vessels (bottom row, green). Direction of blood/lymph flow and color codes representing different tissues are shown below. Developmental time-points, as determined for the valves in the proximal femoral vein and mesenteric lymphatic vessels in mice, are indicated below each stage; E embryonic, P postnatal. Note the presence of uniform smooth muscle coating (brown) in veins prior to valve initiation, while sparse coverage of SMCs is acquired to lymphatic vessels only after valve formation and concomitant remodeling of a primitive vascular plexus to mature collecting vessels. Valve initiation in both veins and lymphatic vessels is characterized by emergence of clusters of cells expressing high levels of Prox1 and Foxc2 transcription factors (dark green nuclei), predominantly near vessel branch points, and followed by formation of leaflets with two layers of endothelial cells expressing Integrin-α9 (light green) attached to Laminin-α5 positive matrix core (red). Bottom confocal micrograph of a dermal collecting lymphatic vessel stained for Laminin-α5 to visualize the extracellular matrix core of the valve leaflet (green) and αSMA to highlight smooth muscle cells around the vessel (red). Scale bar 50 μm
Fig. 3
Model of valve morphogenesis. a Schematic representation of the development of a bicuspid venous valve by Kampmeier in 1927 ([28], reproduced from [27], Copyright (1927), Royal Society Medicine Press, UK). Endothelial layer is shown in white and mesenchymal layer in black. Flow direction is indicated by arrows; t tributary. b Schematic model of lymphatic valve morphogenesis. Some of the key regulators of different stages of valve formation are shown. Green text indicates expression in lymphatic endothelial cells, dark brown smooth muscle cells, and red extracellular matrix components. The initiation of valve formation coincides with the initiation of lymph flow in mesenteric lymphatic vessels. Clusters of endothelial cells expressing high levels of Prox1 and Foxc2 transcription factors (dark green nuclei) emerge at the sites of developing valves, which is followed by deposition of matrix molecules, such as Laminin-α5 (red) and establishment of valve territory via Calcineurin and Connexin signaling. Valve leaflet formation is initiated by the formation of an endothelial cell ring-like constriction and depends on Integrin-α9-mediated assembly of FN-EIIIA matrix. Repulsive signaling between Sema3A and NRP1 maintains valve areas free of smooth muscle cells (SMC, brown), while Calcineurin and Ephrin-B2 signaling regulate the maintenance of valve leaflets
Fig. 4
The molecular identity and morphology of valve endothelial cells. a, b Confocal micrographs of a mesenteric lymphatic vessel (a) and a femoral vein (b) stained for Integrin-α9 and Prox1 to visualize valve endothelial cells (red) and their nuclei (green), respectively. Image (b) reproduced from [32]), Copyright (2011), The Journal of Clinical Investigation, USA). Asterisks in (b) indicate a Prox1+ lymphatic vessel running in parallel of the vein. c–f Scanning electron microscope micrographs of a venous valve, showing different endothelial cell phenotypes (regarding cell shape and alignment) in different parts of the valve. Cells on the leaflet show rounded morphology (d) while cells on the inflow side (upstream) of the valve show elongated morphology and align in flow direction (e). The free edges of the leaflets are composed of cells that show transverse orientation and are highly elongated (f, arrows). I inflow, O outflow, L leaflet. Scale bars (a, b) 50 μm, (c, f) 10 μm, (d, e) 5 μm
Similar articles
- Lymphatic collecting vessel maturation and valve morphogenesis.
Vittet D. Vittet D. Microvasc Res. 2014 Nov;96:31-7. doi: 10.1016/j.mvr.2014.07.001. Epub 2014 Jul 12. Microvasc Res. 2014. PMID: 25020266 Review. - Primary and secondary lymphatic valve development: molecular, functional and mechanical insights.
Bazigou E, Wilson JT, Moore JE Jr. Bazigou E, et al. Microvasc Res. 2014 Nov;96:38-45. doi: 10.1016/j.mvr.2014.07.008. Epub 2014 Jul 30. Microvasc Res. 2014. PMID: 25086182 Free PMC article. Review. - Multiple mouse models of primary lymphedema exhibit distinct defects in lymphovenous valve development.
Geng X, Cha B, Mahamud MR, Lim KC, Silasi-Mansat R, Uddin MKM, Miura N, Xia L, Simon AM, Engel JD, Chen H, Lupu F, Srinivasan RS. Geng X, et al. Dev Biol. 2016 Jan 1;409(1):218-233. doi: 10.1016/j.ydbio.2015.10.022. Epub 2015 Nov 2. Dev Biol. 2016. PMID: 26542011 Free PMC article. - Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice.
Bazigou E, Lyons OT, Smith A, Venn GE, Cope C, Brown NA, Makinen T. Bazigou E, et al. J Clin Invest. 2011 Aug;121(8):2984-92. doi: 10.1172/JCI58050. Epub 2011 Jul 18. J Clin Invest. 2011. PMID: 21765212 Free PMC article. - Absence of venous valves in mice lacking Connexin37.
Munger SJ, Kanady JD, Simon AM. Munger SJ, et al. Dev Biol. 2013 Jan 15;373(2):338-48. doi: 10.1016/j.ydbio.2012.10.032. Epub 2012 Nov 7. Dev Biol. 2013. PMID: 23142761 Free PMC article.
Cited by
- Phenomena of Intussusceptive Angiogenesis and Intussusceptive Lymphangiogenesis in Blood and Lymphatic Vessel Tumors.
Díaz-Flores L, Gutiérrez R, González-Gómez M, García MDP, Carrasco-Juan JL, Martín-Vasallo P, Madrid JF, Díaz-Flores L Jr. Díaz-Flores L, et al. Biomedicines. 2024 Jan 23;12(2):258. doi: 10.3390/biomedicines12020258. Biomedicines. 2024. PMID: 38397861 Free PMC article. - Foxc1 and Foxc2 deletion causes abnormal lymphangiogenesis and correlates with ERK hyperactivation.
Fatima A, Wang Y, Uchida Y, Norden P, Liu T, Culver A, Dietz WH, Culver F, Millay M, Mukouyama YS, Kume T. Fatima A, et al. J Clin Invest. 2016 Jul 1;126(7):2437-51. doi: 10.1172/JCI80465. Epub 2016 May 23. J Clin Invest. 2016. PMID: 27214551 Free PMC article. - Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Angiogenesis: Potencial Clinical Application.
Merino-González C, Zuñiga FA, Escudero C, Ormazabal V, Reyes C, Nova-Lamperti E, Salomón C, Aguayo C. Merino-González C, et al. Front Physiol. 2016 Feb 9;7:24. doi: 10.3389/fphys.2016.00024. eCollection 2016. Front Physiol. 2016. PMID: 26903875 Free PMC article. Review. - RASA1-dependent cellular export of collagen IV controls blood and lymphatic vascular development.
Chen D, Teng JM, North PE, Lapinski PE, King PD. Chen D, et al. J Clin Invest. 2019 Jun 11;129(9):3545-3561. doi: 10.1172/JCI124917. J Clin Invest. 2019. PMID: 31185000 Free PMC article. - Differential Expression Patterns of Eph Receptors and Ephrin Ligands in Human Cancers.
Kou CJ, Kandpal RP. Kou CJ, et al. Biomed Res Int. 2018 Feb 28;2018:7390104. doi: 10.1155/2018/7390104. eCollection 2018. Biomed Res Int. 2018. PMID: 29682554 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources