The effect of bifid triple viable on immune function of patients with ulcerative colitis - PubMed (original) (raw)
The effect of bifid triple viable on immune function of patients with ulcerative colitis
Guohua Li et al. Gastroenterol Res Pract. 2012.
Abstract
Objective. To study effect and its mechanism of Bifid Triple Viable for initially treating ulcerative colitis with 5-aminosalicylic acid. Methods. 82 patients, who were firstly diagnosed as ulcerative colitis, were randomized into experiment group (41 cases, treated with Bifid Triple Viable and Etiasa) and control group (41 cases, treated with Etiasa). The clinic symptom score, colon mucosa inflammation score, and some immune indices were detected and compared between two groups before and two months after treatment. Results. Two months after treatment, the clinical symptom score, colon mucosa inflammation score, and IL-1β expression in colon mucosa decreased significantly (P < 0.01), and IL-10 and IgA expressions in colon mucosa increased significantly (P < 0.01). Those differences were more marked in experiment group than control group (P < 0.05). However, peripheral blood T cell subgroup, immunoglobulins, and complements had no significant difference between two groups two months after treatment, but the ratio of peripheral blood CD4+ T cell to CD8+ T cell in experiment group increased more than that in control group (P < 0.05). Conclusion. Bifid Triple Viable contributed to Etiasa to treat ulcerative colitis in inducing remission period, which was perhaps related to affecting the patient's immune function.
Figures
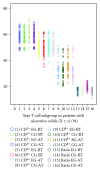
Figure 1
EG stands for experiment group; CG stands for control group; BT stands for “before treatment”. AT stands for “after treatment”. Ratio stands for the tenfold ratio of CD4+T cells to CD8+ T cells. There was no significant difference regarding the average values of T-cell subgroup between two groups before treatment and two months after treatment (P > 0.05). However, the ratio of CD4+ T cell to CD8+ T cell in experiment group two months after treatment increased more than that in control group (P < 0.05).
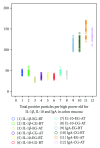
Figure 2
EG stands for experiment group; CG stands for control group; BT stands for “before treatment”. AT stands for “after treatment”. The average number of positive immunoreactivity particles for IgA, IL-1_β_, or IL-10 in colon mucosa was no significant difference before treatment between two groups. Two months after treatment, the average number of positive particles for IgA and IL-10 in colon mucosa in each group increased significantly (P < 0.01). Moreover, the average number of positive particles for IgA and IL-10 in experiment group was more than that in control group (P < 0.01). However, average number of positive particles of IL-1_β_ was opposite to that of IgA and IL-10 (P < 0.05).
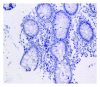
Figure 3
No positive particle (1 × 100).
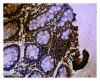
Figure 4
Positive particle for anti-IL-10 (1 × 40).
Figure 5
Positive particle for anti-IgA (1 × 100).
Figure 6
Positive particle for anti-IL-1_β_ (1 × 40).
Similar articles
- Supplemental bifid triple viable capsule treatment improves inflammatory response and T cell frequency in ulcerative colitis patients.
Li S, Yin Y, Xiao D, Zou Y. Li S, et al. BMC Gastroenterol. 2021 Aug 4;21(1):314. doi: 10.1186/s12876-021-01887-2. BMC Gastroenterol. 2021. PMID: 34348654 Free PMC article. - Effects of mesalazine combined with bifid triple viable on intestinal flora, immunoglobulin and levels of cal, MMP-9, and MPO in feces of patients with ulcerative colitis.
Jiang XE, Yang SM, Zhou XJ, Zhang Y. Jiang XE, et al. Eur Rev Med Pharmacol Sci. 2020 Jan;24(2):935-942. doi: 10.26355/eurrev_202001_20079. Eur Rev Med Pharmacol Sci. 2020. PMID: 32017001 - Efficacy of mesalazine in combination with bifid triple viable capsules on ulcerative colitis and the resultant effect on the inflammatory factors.
Huang M, Chen Z, Lang C, Chen J, Yang B, Xue L, Zhang Y. Huang M, et al. Pak J Pharm Sci. 2018 Nov;31(6(Special)):2891-2895. Pak J Pharm Sci. 2018. PMID: 30630805 Clinical Trial. - [Efficacy of probiotics on ulcerative colitis and its mechanism].
Li K, Zhang CF, Xia YH, Li ZJ, Han Y. Li K, et al. Zhonghua Wei Chang Wai Ke Za Zhi. 2013 Apr;16(4):336-9. Zhonghua Wei Chang Wai Ke Za Zhi. 2013. PMID: 23608794 Clinical Trial. Chinese. - 5-Aminosalicylic acid, a specific drug for ulcerative colitis.
Hauso Ø, Martinsen TC, Waldum H. Hauso Ø, et al. Scand J Gastroenterol. 2015 Aug;50(8):933-41. doi: 10.3109/00365521.2015.1018937. Epub 2015 Mar 2. Scand J Gastroenterol. 2015. PMID: 25733192 Review.
Cited by
- Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease.
Ghouri YA, Richards DM, Rahimi EF, Krill JT, Jelinek KA, DuPont AW. Ghouri YA, et al. Clin Exp Gastroenterol. 2014 Dec 9;7:473-87. doi: 10.2147/CEG.S27530. eCollection 2014. Clin Exp Gastroenterol. 2014. PMID: 25525379 Free PMC article. Review. - Probiotics for inflammatory bowel disease: Is there sufficient evidence?
Ma Y, Yang D, Huang J, Liu K, Liu H, Wu H, Bao C. Ma Y, et al. Open Life Sci. 2024 Apr 5;19(1):20220821. doi: 10.1515/biol-2022-0821. eCollection 2024. Open Life Sci. 2024. PMID: 38585636 Free PMC article. Review. - Molecular mechanisms by which casein glycomacropeptide maintains internal homeostasis in mice with experimental ulcerative colitis.
Cui Y, Zhu C, Ming Z, Cao J, Yan Y, Zhao P, Pang G, Deng Z, Yao Y, Chen Q. Cui Y, et al. PLoS One. 2017 Jul 10;12(7):e0181075. doi: 10.1371/journal.pone.0181075. eCollection 2017. PLoS One. 2017. PMID: 28700735 Free PMC article. - Efficacy and Safety of Probiotics Combined With Traditional Chinese Medicine for Ulcerative Colitis: A Systematic Review and Meta-Analysis.
Hu Y, Ye Z, She Y, Li L, Wu M, Qin K, Li Y, He H, Hu Z, Yang M, Lu F, Ye Q. Hu Y, et al. Front Pharmacol. 2022 Mar 7;13:844961. doi: 10.3389/fphar.2022.844961. eCollection 2022. Front Pharmacol. 2022. PMID: 35321324 Free PMC article. Review. - Efficacy and safety of probiotics in IBD: An overview of systematic reviews and updated meta-analysis of randomized controlled trials.
Estevinho MM, Yuan Y, Rodríguez-Lago I, Sousa-Pimenta M, Dias CC, Barreiro-de Acosta M, Jairath V, Magro F. Estevinho MM, et al. United European Gastroenterol J. 2024 Sep;12(7):960-981. doi: 10.1002/ueg2.12636. Epub 2024 Aug 6. United European Gastroenterol J. 2024. PMID: 39106167 Free PMC article.
References
- Ouyang Q, Tandon R, Goh KL, Ooi CJ, Ogata H, Fiocchi C. The emergence of inflammatory bowel disease in the Asian Pacific region. Current Opinion in Gastroenterology. 2005;21(4):408–413. - PubMed
- Png CW, Lindén SK, Gilshenan KS, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. American Journal of Gastroenterology. 2010;105(11):2420–2428. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous