Anticancer activity of MPT0E028, a novel potent histone deacetylase inhibitor, in human colorectal cancer HCT116 cells in vitro and in vivo - PubMed (original) (raw)
Anticancer activity of MPT0E028, a novel potent histone deacetylase inhibitor, in human colorectal cancer HCT116 cells in vitro and in vivo
Han-Li Huang et al. PLoS One. 2012.
Erratum in
- PLoS One. 2012;7(9). doi: 10.1371/annotation/ab4fff87-6a32-4718-aa4c-91658f164b8d. Huang, Han-Lin [corrected to Huang, Han-Li]
Abstract
Recently, histone deacetylase (HDAC) inhibitors have emerged as a promising class of drugs for treatment of cancers, especially subcutaneous T-cell lymphoma. In this study, we demonstrated that MPT0E028, a novel N-hydroxyacrylamide-derived HDAC inhibitor, inhibited human colorectal cancer HCT116 cell growth in vitro and in vivo. The results of NCI-60 screening showed that MPT0E028 inhibited proliferation in both solid and hematological tumor cell lines at micromolar concentrations, and was especially potent in HCT116 cells. MPT0E028 had a stronger apoptotic activity and inhibited HDACs activity more potently than SAHA, the first therapeutic HDAC inhibitor proved by FDA. In vivo murine model, the growth of HCT116 tumor xenograft was delayed and inhibited after treatment with MPT0E028 in a dose-dependent manner. Based on in vivo study, MPT0E028 showed stronger anti-cancer efficacy than SAHA. No significant body weight difference or other adverse effects were observed in both MPT0E028-and SAHA-treated groups. Taken together, our results demonstrate that MPT0E028 has several properties and is potential as a promising anti-cancer therapeutic drug.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
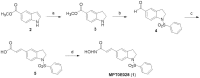
Figure 1. The synthesis of MPT0E028. Reagents and conditions.
(a) NaBH3CN, AcOH, 0°C–r.t.; (b) (i) benzenesulfonyl chloride, pyridine, reflux; (ii) LiAlH4, THF, 0°C–r.t.; (iii) pyridinium dichromate (PDC), molecular sieves, CH2Cl2, r.t.; (c) (i) methyl(triphenylphosphorylidene)acetate, CH2Cl2, r.t.; (ii) 1 M LiOH(aq), dioxane, 40°C; (d) (i) NH2OTHP, PYBOP, Et3N, DMF, r.t.; (iii) trifluoroacetic acid, CH3OH, r.t. Abbreviations: NaBH3CN, sodium cyanoborohydride; AcOH, acetyl acid; LiAlH4 (LAH), lithium aluminium hydride; THF, tetrahydrofuran; PDC, pyridinium dichromate; NH2OTHP, _O_-(tetrahydro-2H-pyran-2-yl)hydroxylamine; PYBOP, benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate; Et3N, triethylamine; DMF, dimethylformamide; TFA, trifluoroacetic acid.
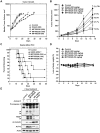
Figure 2. The effects of MPT0E028 on cell growth and cell cycle progression in human HCT116 cells.
(A) Concentration-dependent effect of MPT0E028 and SAHA on cell growth. HCT116 cells were incubated without or with the indicated concentrations of MPT0E028 or SAHA for 48 h. Cell growth was evaluated by SRB assay. Data were expressed as mean±S.E.M. of at least 3 independent experiments. (B) Concentration-dependent effects of MPT0E028 and SAHA on cell cycle progression. HCT116 cells were treated without or with the indicated concentrations of MPT0E028 or SAHA for 24 h and were analyzed by flow cytometry for cell cycle distribution. (C, D) Data shown are the means of at least 3 independent experiments. (E) Time-dependent effects of MPT0E028 and SAHA on subG1 population. HCT116 cells were treated without or with 1 µM MPT0E028 or SAHA for the indicated time interval and were analyzed by flow cytometry for subG1 population. (F) MPT0E028 induced-caspase 3 and PARP activation. HCT116 cells were treated without or with the indicated concentration of MPT0E028 or SAHA for 24 h subject to western blot for caspase 3 and PARP analysis. (G) MPT0E028 induced casepase-dependent cell apoptosis. HCT116 cells were treated without or with 3 µM MPTE028, SAHA or 20 µM z-VAD-fmk for 24 h and subjected to western blot for caspase 3 and PARP analysis.
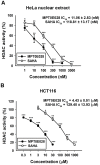
Figure 3. Inhibition of HDACs activity by MPT0E028 and SAHA.
(A) Inhibition of HDACs activity in HeLa nuclear extracts. Data were expressed as the mean of at least 3 independent experiments. (B) Inhibition of total HDAC activity by MPT0E028 and SAHA. HCT116 cells were treated with the indicated concentrations of MPT0E028 and SAHA for 24 h, and the nuclear proteins were isolated to determine the inhibition of total HDAC enzyme activity. Data are expressed as the mean±S.E.M. of at least 3 independent experiments.
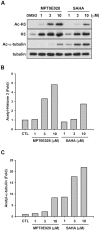
Figure 4. Effect of MPT0E028 and SAHA on α-tubulin and histone H3 acetylation.
(A) HCT116 cells were treated with MPT0E028 and SAHA for 24 h at the indicated concentrations. Cell lysates were prepared and subjected to SDS-PAGE and immunoblotting using acetyl-histone H3, histone H3, acetyl-α-tubulin, and α-tubulin antibodies. Quantitative analysis of western blot with ImageQuant (Molecular Dynamics, USA); acetyl-histone H3 (B) and acetyl α-tubulin (C) were analyzed in HCT116 cells.
Figure 5. The effect of MPT0E028 on the growth of HCT116 cells in vivo.
All tumors grew to the 1,200-mm3 endpoint volume. (A) Tumors were measured regularly and growth delay was calculated for treatment groups relative to control tumors (TGD). (B) Kaplan-Meier survival analysis was based on the tumor growth endpoint. (C) Inhibition of tumor growth curves represented a mean±SEM and percentage change in mean tumor volume (percent TGI). (D) Body weights were measured daily during the first week and then 2 times per week. The body weight ratio was calculated relative to the baseline measurement. (E) In vivo effect of MPT0E028 on the expression of caspase 3, PARP, acetyl-histone H3 and acetyl-µ-tubulin in HCT116 xenograft tumors as determined by western blotting.
Similar articles
- Synergistic interaction between the HDAC inhibitor, MPT0E028, and sorafenib in liver cancer cells in vitro and in vivo.
Chen CH, Chen MC, Wang JC, Tsai AC, Chen CS, Liou JP, Pan SL, Teng CM. Chen CH, et al. Clin Cancer Res. 2014 Mar 1;20(5):1274-1287. doi: 10.1158/1078-0432.CCR-12-3909. Epub 2014 Feb 11. Clin Cancer Res. 2014. PMID: 24520095 Free PMC article. - Novel oral histone deacetylase inhibitor, MPT0E028, displays potent growth-inhibitory activity against human B-cell lymphoma in vitro and in vivo.
Huang HL, Peng CY, Lai MJ, Chen CH, Lee HY, Wang JC, Liou JP, Pan SL, Teng CM. Huang HL, et al. Oncotarget. 2015 Mar 10;6(7):4976-91. doi: 10.18632/oncotarget.3213. Oncotarget. 2015. PMID: 25669976 Free PMC article. - Design, synthesis, and biological evaluation of indole-based hydroxamic acid derivatives as histone deacetylase inhibitors.
Jiang BE, Hu J, Liu H, Liu Z, Wen Y, Liu M, Zhang HK, Pang X, Yu LF. Jiang BE, et al. Eur J Med Chem. 2022 Jan 5;227:113893. doi: 10.1016/j.ejmech.2021.113893. Epub 2021 Oct 2. Eur J Med Chem. 2022. PMID: 34656899 - Novel β-Carboline/Hydroxamic Acid Hybrids Targeting Both Histone Deacetylase and DNA Display High Anticancer Activity via Regulation of the p53 Signaling Pathway.
Ling Y, Xu C, Luo L, Cao J, Feng J, Xue Y, Zhu Q, Ju C, Li F, Zhang Y, Zhang Y, Ling X. Ling Y, et al. J Med Chem. 2015 Dec 10;58(23):9214-27. doi: 10.1021/acs.jmedchem.5b01052. Epub 2015 Nov 24. J Med Chem. 2015. PMID: 26555243 - In vivo Anticancer Potential of Hydroxamic Acid Derivatives.
Li H, Gong Y, Zhong Q. Li H, et al. Curr Top Med Chem. 2021;21(19):1737-1755. doi: 10.2174/1568026621666210813105240. Curr Top Med Chem. 2021. PMID: 34392823 Review.
Cited by
- The HDAC inhibitor, MPT0E028, enhances erlotinib-induced cell death in EGFR-TKI-resistant NSCLC cells.
Chen MC, Chen CH, Wang JC, Tsai AC, Liou JP, Pan SL, Teng CM. Chen MC, et al. Cell Death Dis. 2013 Sep 19;4(9):e810. doi: 10.1038/cddis.2013.330. Cell Death Dis. 2013. PMID: 24052078 Free PMC article. - Synergistic interaction between the HDAC inhibitor, MPT0E028, and sorafenib in liver cancer cells in vitro and in vivo.
Chen CH, Chen MC, Wang JC, Tsai AC, Chen CS, Liou JP, Pan SL, Teng CM. Chen CH, et al. Clin Cancer Res. 2014 Mar 1;20(5):1274-1287. doi: 10.1158/1078-0432.CCR-12-3909. Epub 2014 Feb 11. Clin Cancer Res. 2014. PMID: 24520095 Free PMC article. - A chemical probe toolbox for dissecting the cancer epigenome.
Shortt J, Ott CJ, Johnstone RW, Bradner JE. Shortt J, et al. Nat Rev Cancer. 2017 Feb 23;17(3):160-183. doi: 10.1038/nrc.2016.148. Nat Rev Cancer. 2017. PMID: 28228643 Review. - Novel oral histone deacetylase inhibitor, MPT0E028, displays potent growth-inhibitory activity against human B-cell lymphoma in vitro and in vivo.
Huang HL, Peng CY, Lai MJ, Chen CH, Lee HY, Wang JC, Liou JP, Pan SL, Teng CM. Huang HL, et al. Oncotarget. 2015 Mar 10;6(7):4976-91. doi: 10.18632/oncotarget.3213. Oncotarget. 2015. PMID: 25669976 Free PMC article. - In vitro and in vivo anti-tumour effects of MPT0B014, a novel derivative aroylquinoline, and in combination with erlotinib in human non-small-cell lung cancer cells.
Tsai AC, Pai HC, Wang CY, Liou JP, Teng CM, Wang JC, Pan SL. Tsai AC, et al. Br J Pharmacol. 2014 Jan;171(1):122-33. doi: 10.1111/bph.12427. Br J Pharmacol. 2014. PMID: 24116948 Free PMC article.
References
- Khan O, La Thangue NB (2012) HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol 90: 85–94. - PubMed
- Beumer J, Tawbi H (2010) Role of histone deacetylases and their inhibitors in cancer biology and treatment. Curr Clin Pharmacol 5: 196–208. - PubMed
- Prince HM, Bishton MJ, Harrison SJ (2009) Clinical studies of histone deacetylase inhibitors. Clin Cancer Res 15: 3958–3969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
The study was supported by a grant from the National Science Council of Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical