Augmented generation of protein fragments during wakefulness as the molecular cause of sleep: a hypothesis - PubMed (original) (raw)
Augmented generation of protein fragments during wakefulness as the molecular cause of sleep: a hypothesis
Alexander Varshavsky. Protein Sci. 2012 Nov.
Abstract
Despite extensive understanding of sleep regulation, the molecular-level cause and function of sleep are unknown. I suggest that they originate in individual neurons and stem from increased production of protein fragments during wakefulness. These fragments are transient parts of protein complexes in which the fragments were generated. Neuronal Ca²⁺ fluxes are higher during wakefulness than during sleep. Subunits of transmembrane channels and other proteins are cleaved by Ca²⁺-activated calpains and by other nonprocessive proteases, including caspases and secretases. In the proposed concept, termed the fragment generation (FG) hypothesis, sleep is a state during which the production of fragments is decreased (owing to lower Ca²⁺ transients) while fragment-destroying pathways are upregulated. These changes facilitate the elimination of fragments and the remodeling of protein complexes in which the fragments resided. The FG hypothesis posits that a proteolytic cleavage, which produces two fragments, can have both deleterious effects and fitness-increasing functions. This (previously not considered) dichotomy can explain both the conservation of cleavage sites in proteins and the evolutionary persistence of sleep, because sleep would counteract deleterious aspects of protein fragments. The FG hypothesis leads to new explanations of sleep phenomena, including a longer sleep after sleep deprivation. Studies in the 1970s showed that ethanol-induced sleep in mice can be strikingly prolonged by intracerebroventricular injections of either Ca²⁺ alone or Ca²⁺ and its ionophore (Erickson et al., Science 1978;199:1219-1221; Harris, Pharmacol Biochem Behav 1979;10:527-534; Erickson et al., Pharmacol Biochem Behav 1980;12:651-656). These results, which were never interpreted in connection to protein fragments or the function of sleep, may be accounted for by the FG hypothesis about molecular causation of sleep.
Copyright © 2012 The Protein Society.
Figures
Figure 1
Generation, targeting and degradation of protein fragments, and remodeling of cleaved protein complexes. This diagram illustrates a multistage process that is expected to occur in a great variety of structural contexts and is a part of reactions encompassed by the fragment generation (FG) hypothesis. The process begins with a proteolytic cleavage (lightning arrow) of an oligomeric (in this example, heterodimeric) protein by a nonprocessive protease such as, for example, calpain or caspase. The process continues with the subunit-selective degradation of a resulting C-terminal fragment either by the Arg/N-end rule pathway (in this example) or by another proteolytic pathway, and ends with reconstitution of the initial heterodimer. Such processes can involve both “soluble” and transmembrane proteins (Figs. 3–6). The arrangement of two subunits in the heterodimer is (arbitrarily) antiparallel, with N-termini and C-termini denoted by “N” and “C,” respectively. The rate constants k1–k6 illustrate the possibility of independent regulation of specific reaction steps. In this example, the N-terminal residue of the C-terminal fragment (it remains associated with the other subunit of heterodimer) is Gln (Q), a tertiary destabilizing residue [Fig. 2(A)]. The C-terminal fragment is sequentially modified by the Ntaq1 NQ-amidase and the Ate1 R-transferase (specific enzymes of the Arg/N-end rule pathway) [Fig. 2(A)], followed by polyubiquitylation of the fragment by N-recognins (ubiquitin ligases of the Arg/N-end rule pathway) such as the UBR1 E3 (only E3 component of the holoenzyme ligase is shown) and the proteasome-mediated degradation of the targeted C-terminal fragment. For illustrative purposes, each of two subunits is shown to consist of two domains. The N-terminal fragment of the cleaved subunit may also be targeted for degradation, either by the Ac/N-end rule pathway (via an Ac/N-degron of the fragment) in this example, [Fig. 2(B)], or by another proteolytic pathway. See Fig. 2 and the main text for additional details. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
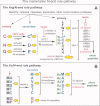
Figure 2
The mammalian N-end rule pathway.,,, N-terminal residues are indicated by single-letter abbreviations for amino acids. A yellow oval denotes the rest of a protein substrate. E3 ubiquitin ligases of the N-end rule pathway are called N-recognins. A: The Arg/N-end rule pathway. “Primary,” “secondary,” and “tertiary” denote mechanistically distinct subsets of destabilizing N-terminal residues. C* denotes oxidized N-terminal Cys, either Cys-sulfinate or Cys-sulfonate, produced in vivo by reactions that require nitric oxide (NO) and oxygen. Oxidized N-terminal Cys is arginylated by _ATE1_-encoded isoforms of arginyl-tRNA-protein transferase (R-transferase), which also arginylates N-terminal Asp (D) and Glu (E). N-terminal Asn (N) and Gln (Q) are deamidated by the _NTAN1_-encoded NtN-amidase and the _NTAQ1_-encoded NtQ-amidase, respectively. In addition to the binding sites that recognize primary destabilizing N-terminal residues, the UBR1, UBR2, UBR4 and UBR5 (EDD) N-recognins contain binding sites for substrates (denoted by a larger oval) that lack N-degrons and have internal (non-N-terminal) degradation signals., Polyubiquitylated Arg/N-end rule substrates are degraded to short peptides by the 26S proteasome. Hemin (Fe3+-heme) binds to R-transferase, inhibits its arginylation activity and accelerates its in vivo degradation.,, Hemin also binds to UBR-type N-recognins. Regulated degradation of specific proteins by the Arg/N-end rule pathway mediates the sensing of heme, NO, and oxygen; the elimination of misfolded proteins; the regulation of DNA repair; the fidelity of chromosome cohesion/segregation; the signaling by G proteins; the control of peptide import; the regulation of apoptosis, meiosis, viral infections, fat metabolism, cell migration, actin filaments, cardiovascular development, spermatogenesis, neurogenesis and memory; the functioning of adult organs, including the brain, muscle, testis and pancreas; and many functions in plants (Refs. –,,,,,,, and references therein). B: The Ac/N-end rule pathway. Although it is clear that this pathway is present in all or most eukaryotes, it has been characterized, thus far, only in yeast. This diagram illustrates the mammalian Ac/N-end rule pathway through an extrapolation from its S. cerevisiae version. Red arrow on the left indicates the removal of N-terminal Met by Met-aminopeptidases (MetAPs). This Met residue is retained if a residue at position 2 is nonpermissive (too large) for MetAPs. If the retained N-terminal Met or N-terminal Ala, Val, Ser, Thr, and Cys are followed by acetylation-permissive residues, the above N-terminal residues are Nt-acetylated by ribosome-associated Nt-acetylases. The resulting N-degrons are called Ac/N-degrons. The term “secondary” refers to the necessity of modification (Nt-acetylation) of a destabilizing N-terminal residue before a protein can be recognized by a cognate N-recognin. Although the second-position Gly or Pro residues can be made N-terminal by MetAPs, few proteins with N-terminal Gly or Pro are Nt-acetylated. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3
Calpain-generated C-terminal fragments of mammalian proteins that are either identified or predicted substrates of the Arg/N-end rule pathway. Each entry cites, on the left, a C-terminal (Ct) fragment and its N-terminal (Nt) residue (in red, using three-letter abbreviations for amino acids), followed by a brief description of the full-length (uncleaved) precursor protein. The right side of each entry shows the cleavage site of a full-length protein, using single-letter abbreviations for amino acids. An enlarged residue name, in red (preceded by an arrowhead denoting the cleavage site), indicates the P1′ residue, that is, the residue that becomes Nt upon the cleavage. Unless stated otherwise, the residue numbers are of human proteins. Two indicated residue numbers are the number of the first shown residue of a full-length protein and the number of its last residue, respectively. Most proteins on the list are present at least in neurons. These 34 proteins encompass the bulk of the previously characterized calpain substrates whose cleavage sites have been mapped and whose Ct fragments bear Nt residues that can be recognized by the Arg/N-end rule pathway [Fig. 2(A)]. Not shown are ∼20 calpain-generated, previously mapped Ct fragments bearing Nt residues that are not recognized by the Arg/N-end rule pathway. The Nt residues of this class, including Ala, Ser, and Thr, can be recognized by the Ac/N-end rule pathway [Fig. 2(B)] if these residues can be Nt-acetylated after having become Nt through a calpain-mediated cleavage. Whether these post-translationally generated N-terminal residues can be efficaciously Nt-acetylated in vivo remains to be determined. In addition, there are ∼40 other identified mammalian calpain substrates in which the exact locations of cleavage sites are unknown. Two calpain-generated Ct fragments, Asp-BCLXL (#16) and Arg-BID (#17), have been shown to be short-lived substrates of the Arg/N-end rule pathway. Other Ct fragments on this list are predicted Arg/N-end rule substrates. The recent finding that 10 out of 10 Ct fragments (generated by caspases or calpains) that were predicted to be Arg/N-rule substrates were actually found to be such suggests that most Ct fragments on the present list are also degraded by the Arg/N-end rule pathway. Calpain-generated fragments: #1. Tyr-mGluR1α is the Ct fragment of the mGluR1α subunit of the transmembrane metabotropic glutamate receptor. Receptors containing the calpain-truncated mGluR1α subunit could elevate cytosolic Ca2+ but could not activate PI3K-Akt signaling pathways, in contrast to uncleaved receptors., #2. Leu-NR2A is the Ct-fragment of the NR2A subunit of the transmembrane ionotropic glutamate receptor (NMDAR). The NR2B subunit of NMDAR can also be cleaved by calpains. Ct fragments of NR2A and NR2B contain domains required for the association of these subunits with synaptic proteins. NMDAR receptors lacking the Ct region of NR2A could function as glutamate-gated Ca2+ channels but the intracellular traffic of cleaved receptors and their electrophysiological properties were altered. #3. Lys-ATP2B2 is the Ct fragment of the transmembrane ATP2B2 plasma membrane Ca2+ pump (PMCA) that ejects Ca2+ from cells. This pump is activated either by the binding of Ca2+/calmodulin or by the calpain-mediated truncation of ATP2B2 that generates the Lys-ATP2B2 fragment and thereby activates the pump. #4. Gln-RYR1 is the Ct fragment of the RYR1 ryanodine receptor, a Ca2+ channel in the ER that mediates the efflux of Ca2+ from the ER into the cytosol. Calpain-mediated cleavage of RYR1 increases Ca2+ efflux. #5. Gln-EGFR is one of the calpain-generated Ct fragments of the transmembrane epidermal growth factor (EGF) receptor protein kinase. Remarkably, all seven calpain cleavage sites in the cytosol-exposed domain of the 170-kDa EGFR contain P1′ residues (which become Nt upon a cleavage) that are destabilizing in the Arg/N-end rule [Fig. 2(A)]. #6. Asn-Cav1.1 is the Ct fragment of the voltage-gated transmembrane Ca2+ channel. This (apparently) calpain-generated fragment is noncovalently associated with the rest of the channel and can inhibit its activity. Upon dissociation from the channel, the Asn-Cav1.1 fragment migrates to the nucleus and functions as a transcriptional regulator.,,, #7. Arg-GlyT1A is the Ct fragment of the transmembrane GlyT1A glycine transporter. Another Gly transporter, GlyT1B, is also cleaved by calpains, yielding the Arg-GlyT1B fragment. These Ct fragments are still active as transporters but are impaired in their ability to remove Gly (an inhibitory neurotransmitter) from synaptic clefts. #8. Leu-RAD21 is the Ct-fragment of the SCC1/RAD21 subunit of the chromosome-associated cohesin complex. Calpain-mediated generation of Leu-RAD21 contributes to the control of chromosome cohesion/segregation, together with processes that include the separase-mediated cleavage of the same RAD21 subunit,,, [see also Fig. 6(F)]. #9. Lys-cortactin is the Ct fragment of cortactin, an actin-binding protein that regulates actin polymerization. #10. Leu-vimentin is the Ct fragment of vimentin, a component of intermediate filaments. #11. Arg-dystrophin is the Ct fragment of a major cytoskeletal protein in the skeletal muscle. #12. Gln-talin is the Ct fragment of talin, an adaptor protein that interacts with the integrin family of cell adhesion transmembrane proteins.,, #13. Leu-NF2 is the Ct fragment of NF2 (merlin), a tumor suppressor and cytoskeletal protein. Loss-of-function NF2 mutants result in autosomal-dominant neurofibromatosis, a predisposition to specific kinds of brain tumors [see also Fig. 6(E)]. #14. Leu-troponin T2 is the Ct fragment of the cardiac troponin T that is produced by calpain-1 from the troponin-containing cardiac myofibril complex. #15. Glu-BAK is the Ct fragment of the proapoptotic regulator BAK. Glu-BAK is generated by calpain-1 in vitro and may be formed in vivo as well. #16. Asp-BCLXL is the Ct fragment of the BCLXL antiapoptotic protein. In contrast to its full-length precursor, the Asp-BCLXL fragment is proapoptotic, and has been shown to be a short-lived substrate of the Arg/N-end rule pathway. #17. Arg-BID is the Ct fragment of the proapoptotic BID regulator. The Arg-BID fragment is also proapoptotic, and in addition a short-lived Arg/N-end rule substrate. #18. Asn-DSCR1 (RCAN1) is the Ct fragment of the Down syndrome critical region 1 protein DSCR1, which binds to Raf1, inhibits the phosphatase activity of calcineurin, and enhances its degradation. Calpain-generated Asn-DSCR1 does not bind to the Raf1 kinase. #19. Arg-c-FOS is the Ct fragment of the c-FOS transcriptional regulator. c-FOS is targeted for degradation through more than one degron, including the path that includes the cleavage by calpains and predicted degradation of Arg-c-FOS by the Arg/N-end rule pathway. #20. Arg-MEF2D is the Ct fragment of the MEF2D myocyte enhancer factor 2D, a transcriptional regulator that contributes to neuronal survival, development, and synaptic plasticity. #21. Leu-STEP33 is the Ct fragment of the striatal-enriched STEP61 phosphatase, a brain-specific Tyr-phosphatase whose substrates include the MAPK-family kinases ERK1/2 and p38. Calpain-generated Leu-STEP33 fragment lacks phosphatase activity. #22. Leu-β-catenin is the Ct-fragment of β-catenin, a conditionally short-lived cytoskeletal protein and transcriptional regulator. The Leu-β-catenin fragment is a nuclear protein that activates specific genes in conjunction with other transcription factors. #23. Arg-IGFBP2 is the Ct fragment of the insulin-like growth factor binding protein-2 that is cleaved by calpain-2 at least in vitro. #24. Glu-Iκ-Bα is the Ct fragment of the Iκ-Bα subunit of the autoinhibited NF-κB–Iκ-Bα complex in which the NF-κB transcriptional regulator is inhibited by Iκ-Bα. The Iκ-Bα subunit is targeted for degradation either through a conditional phosphodegron or through the calpain-mediated cleavage which produces Glu-Iκ-Bα, predicted to be an Arg/N-end rule substrate. #25. Phe-PKCγ is the Ct fragment of PKCγ, a Ser/Thr kinase of the PKC family. The Phe-PKCγ fragment is constitutively active as a kinase, because it lacks the regulatory Nt domain of the full-length PKCγ kinase. #26. Leu-CAMK-IV is the Ct fragment of the Ca2+/calmodulin-dependent kinase-IV. This fragment lacks kinase activity. #27. Lys-PKCα is the Ct fragment of PKCα, a broadly expressed Ser/Thr kinase of the PKC family. Being catalytically active but no longer controlled by the regulatory Nt domain of the full-length PKCα, the Lys-PKCα fragment can be toxic, for example, upon its formation in an ischemic heart. #28. Arg-p39 is the Ct fragment of the p39 activator of the Cdk5 protein kinase. The indicated cleavage site is located immediately downstream of two other closely spaced (and strongly conserved) calpain cleavage sites in p39. A cleavage at any one of these sites yields a predicted Arg/N-end rule substrate [see Fig. 6(H)]. #29. Lys-GAD65-2 is the Ct fragment of the glutamic acid decarboxylase-65-2 (GAD65-2), which is bound to membranes of synaptic vesicles and mediates the synthesis of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) from the excitatory neurotransmitter glutamate. Calpain-generated Lys-GAD65-2 retains the enzymatic activity of uncleaved GAD65-2 but is no longer associated with synaptic vesicles– [see also Fig. 6(G)]. #30. Arg-caspase-9 is the Ct fragment of caspase-9, which can be inactivated by calpains, followed by the (predicted) degradation of the Arg-caspase-9 fragment by the Arg/N-end rule pathway. #31. Leu-calpain-1 is the Ct fragment of human calpain-1. The Leu-calpain-1 fragment is an activated form of this calpain., #32. Asp-calpain (reg. subunit) is the Ct fragment of the calpain regulatory subunit that is cleaved by activated calpains., #33. Lys-calpain-2 is the Ct fragment of human calpain-2, an activated form of this calpain.– #34. Asn-calpain-B is the calpain-generated Ct fragment of one of two major D. melanogaster calpains and an activated form of this calpain. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 4
Caspase-generated C-terminal fragments of mammalian proteins that are either identified or predicted substrates of the Arg/N-end rule pathway. The 30 caspase substrates cited in this list are a small fraction of ∼1000 known and mapped mammalian caspase substrates (see the main text). For designations, including residue numbering, see the legend of Fig. 3. The first eight caspase-generated, proapoptotic Ct fragments are the recently examined and confirmed short-lived substrates of the Arg/N-end rule pathway. #1. Cys-RIPK1 is the proapoptotic Ct fragment of the RIPK1 kinase, a regulator of apoptosis, necroptosis, and other processes, including antiviral responses that do not involve cell death,– [see also Fig. 6(A)]. #2. Cys-TRAF1 is the proapoptotic Ct fragment of TRAF1, which functions to minimize the activation of caspase-8 and other proapoptotic reactions, in part by contributing to upregulation of the antiapoptotic NF-κB regulon., #3. Asp-BRCA1 is the proapoptotic Ct fragment of BRCA1, a RING-type E3 ubiquitin ligase that functions as a tumor suppressor and participates in DNA repair, cell-cycle regulation, transcriptional control, and other processes, [see also Fig. 6(B)]. #4. Leu-LIMK1 is the proapoptotic Ct fragment of LIMK1, a Ser/Thr kinase that functions, in particular, as a downstream effector of Rho signaling pathways and regulator of actin dynamic. #5. Tyr-NEDD9 is the proapoptotic Ct fragment of NEDD9, a scaffolding protein whose functions include cell attachment, migration, and mitotic control. #6. Arg-BIMEL is the proapoptotic Ct fragment of BIMEL a regulator of apoptosis. #7. Asp-EPHA4 is the proapoptotic Ct fragment of EPHA4, a member of the family of more than 10 mammalian “dependence” receptors (DpRs). These structurally distinct receptors are functionally analogous because of their ability to mediate two opposite physiological outcomes. In the presence of its cognate ligand, a DpR receptor activates signaling pathways that mediate cell survival, migration, proliferation, or differentiation. In the absence of its ligand, a dependence receptor produces a proapoptotic signal, often through the formation, by caspases or other nonprocessive proteases, of a C-terminal proapoptotic fragment that functions in the cytosol and/or the nucleus., #8. Tyr-MET is the proapoptotic Ct fragment of MET, another dependence receptor, with functions in embryonic development and organ formation., The next six caspase-generated proapoptotic Ct fragments are predicted substrates of the Arg/N-end rule pathway. #9. Asn-PKCδ is the proapoptotic Ct fragment of the PKCδ protein kinase. #10. Lys-PKCδ is the proapoptotic Ct fragment of the PKCδ protein kinase. #11. Trp-ETK is the proapoptotic Ct fragment of the ETK/BMC tyrosine kinase, a member of the Btk/Tek kinase family. #12. Gln-SLK is the proapoptotic Ct fragment of SLK, a STE20-related protein kinase that plays a role in regulation of actin fibers. #13. Ile-HPK1 is the proapoptotic Ct fragment of HPK1, the a STE20-related protein kinase whose functions include stimulation of the stress-activated protein kinases SAPKs/JNKs and the NF-κB transcriptional regulon. #14. Ile-MLH1 is the proapoptotic Ct fragment of the MLH1 DNA mismatch repair protein. The next 16 caspase-generated Ct fragments and predicted substrates of the Arg/N-end rule pathway that are not necessarily proapoptotic. These likely Arg/N-end rule substrates are cited to illustrate the remarkable diversity of their precursor proteins. #15. Tyr-CYLD is the Ct fragment of a deubiquitylase that regulates apoptosis and necroptosis [see also Fig. 6(D)[. #16. Leu-p21Cip1/Waf1 is the Ct fragment of p21Cip1/Waf, an inhibitor of cell division. #17. Arg-IP3R is the Ct fragment of the inositol 1,4,5-triphosphate receptor. #18. Asn-LMN1 is the Ct fragment of lamin-A, a component of nuclear lamina. #19. Arg-ETS-1 is the Ct fragment of a transcription factor. #20. Tyr-TOP1 is the Ct fragment of type I DNA topoisomerase. #21. Leu-MEFD2 is the Ct fragment of a transcription factor. #22. Asn-DNA-PK is the Ct fragment of the DNA-dependent protein kinase. #23. Asn-CAD1 is the Ct fragment of E-cadherin, an adhesion receptor. #24. Gln-synphilin-1 is the Ct fragment of synphilin-1, a ligand of α-synuclein [see also Fig. 6(C)]. #25. Tyr-ACINUS is the Ct fragment of a mediator of apoptotic chromatin condensation. #26. Lys-PLECTIN is the Ct fragment of a cytoskeletal protein. #27. Cys-CCNE1 is the Ct fragment of a specific G1/S cyclin. #28. His-PMCA4b is the Ct fragment of a Ca2+ extrusion pump. #29. Asp-CDC42 is the Ct fragment of CDC42, a RAS superfamily member. #30. Tyr-iPLA2 is the Ct fragment of the phospholipase A2 (Ref. 212). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 5
Examples of predicted substrates of the Arg/N-end rule pathway that are produced by nonprocessive proteases other than Met-aminopeptidases, calpains, or caspases. For designations, see the legend of Fig. 3. Although some of the proteases mentioned below (e.g., furin) are usually localized outside the nucleus and cytosol, the cited articles indicate that these proteases can also generate cytosolic or nuclear Ct protein fragments. #1. Furin-mediated cleavage of the bacterial (Bortedella) DNT toxin produces the Glu-DNT fragment, which translocates into the cytosol. #2. Cleavage by γ-secretase generates the Arg-CAD1 fragment of E-cadherin. #3. Another predicted Arg/N-end rule substrate that can be produced by γ-secretase is Gln-ROBO1, the Ct fragment of the transmembrane ROBO1 receptor. #4. Proteinase-3 (myeloblastin), a cytosolic/nuclear protease, produces Arg-p21, the Ct fragment of p21, a cell division inhibitor. #5. The Omi/Htr2 protease can cleave the cIAP1 protein (an antiapoptotic regulator and inhibitor of caspases), yielding the Asn-cIAP1 Ct fragment. #6. An intracellular form of elastase cleaves the PML-RARα oncoprotein fusion, yielding the Tyr-PML-RARα Ct fragment. #7. The aminopeptidase PILSAP removes the first nine residues of the kinase PDK1, yielding the enzymatically active Asp-PDK1 Ct fragment. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 6
Evolutionary conservation of caspase (A–D) and calpain (E–H) cleavage sites, and the conservation of destabilizing activity of P1′ residues in these sites. The caspase cleavage sites in A–D (positions P4–P1) are framed by gray rectangles. The indicated residue numbers, including those of P1′ residues (in color), are of human versions of the full-length proteins. In many caspase and calpain cleavage sites (including those shown in Figs. 3 and 4), P1′ residues are completely conserved at least among vertebrates. By contrast, in some cleavage sites, for example those of BRCA1 in B (Fig. 4 (#3)), synphilin-1 in C (Fig. 4(#24)), CYLD in D (Fig. 4(#15)), and p39 in H (Fig. 3(#28)), the P1′ residues (they are shown in different colors, with predominant identities in red) are not conserved among vertebrates. Remarkably, however, their destabilizing activity in the Arg/N-end rule pathway [Fig. 2(A)] is invariably conserved (see the main text). For brief descriptions of the other cited caspase- or calpain-cleaved proteins RIPK1, NF2, RAD21, and GAD65, see Fig. 4(#1), Fig. 3(#13), Fig. 3(#8), and Fig. 3(#29), respectively. See also the Protein Fragments, Their Generation In Spite of Deleterious Effects, and the Evolutionary Persistence of Sleep section. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 7
Monitoring protein fragments and reacting to their accumulation. This diagram mentions PANX1, a transmembrane protein and conditional ATP release channel (light blue oval near the top on the left) as a possible example of a sensor-effector protein. A cleavage of PANX1 is depicted to induce its activity as an ATP release channel (red semi-oval near the top on the right). By linking fragment formation to upregulation of somnogenic pathways that involve extracellular ATP and cytokines (see the FG Hypothesis and Somnogenic Activity of Cytokines section), this arrangement would make it possible for sleep-regulating pathways to gauge an increased production of many different fragments (green ovals and rectangles) during wakefulness, and to react by increasing the levels of somnogenic compounds, for example, extracellular adenosine, ATP or PGD2. The PANX1 channel was used to illustrate this regulatory arrangement because PANX1 has been shown to be strongly activated upon its cleavage by caspases., See also the Monitoring Protein Fragments and Reacting to Their Accumulation section. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Similar articles
- On the cause of sleep: Protein fragments, the concept of sentinels, and links to epilepsy.
Varshavsky A. Varshavsky A. Proc Natl Acad Sci U S A. 2019 May 28;116(22):10773-10782. doi: 10.1073/pnas.1904709116. Epub 2019 May 13. Proc Natl Acad Sci U S A. 2019. PMID: 31085645 Free PMC article. - Calpain-generated natural protein fragments as short-lived substrates of the N-end rule pathway.
Piatkov KI, Oh JH, Liu Y, Varshavsky A. Piatkov KI, et al. Proc Natl Acad Sci U S A. 2014 Mar 4;111(9):E817-26. doi: 10.1073/pnas.1401639111. Epub 2014 Feb 18. Proc Natl Acad Sci U S A. 2014. PMID: 24550490 Free PMC article. - Neurotoxicity induces cleavage of p35 to p25 by calpain.
Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Lee MS, et al. Nature. 2000 May 18;405(6784):360-4. doi: 10.1038/35012636. Nature. 2000. PMID: 10830966 - Orexin neuronal circuitry: role in the regulation of sleep and wakefulness.
Ohno K, Sakurai T. Ohno K, et al. Front Neuroendocrinol. 2008 Jan;29(1):70-87. doi: 10.1016/j.yfrne.2007.08.001. Epub 2007 Aug 29. Front Neuroendocrinol. 2008. PMID: 17910982 Review. - [Selective stimulations and lesions of the rat brain nuclei as the models for research of the human sleep pathology mechanisms].
Šaponjić J. Šaponjić J. Glas Srp Akad Nauka Med. 2011;(51):85-97. Glas Srp Akad Nauka Med. 2011. PMID: 22165729 Review. Serbian.
Cited by
- Usp14 down-regulation corrects sleep and circadian dysfunction of a Drosophila model of Parkinson's disease.
Favaro M, Mauri S, Bernardo G, Zordan MA, Mazzotta GM, Ziviani E. Favaro M, et al. Front Neurosci. 2024 Aug 5;18:1410139. doi: 10.3389/fnins.2024.1410139. eCollection 2024. Front Neurosci. 2024. PMID: 39161651 Free PMC article. - Mitochondrial autophagy in the sleeping brain.
Mauri S, Favaro M, Bernardo G, Mazzotta GM, Ziviani E. Mauri S, et al. Front Cell Dev Biol. 2022 Aug 24;10:956394. doi: 10.3389/fcell.2022.956394. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36092697 Free PMC article. Review. - Ubiquitin proteasome system in circadian rhythm and sleep homeostasis: Lessons from Drosophila.
Ukita Y, Okumura M, Chihara T. Ukita Y, et al. Genes Cells. 2022 Jun;27(6):381-391. doi: 10.1111/gtc.12935. Epub 2022 Apr 19. Genes Cells. 2022. PMID: 35438236 Free PMC article. Review. - On the cause of sleep: Protein fragments, the concept of sentinels, and links to epilepsy.
Varshavsky A. Varshavsky A. Proc Natl Acad Sci U S A. 2019 May 28;116(22):10773-10782. doi: 10.1073/pnas.1904709116. Epub 2019 May 13. Proc Natl Acad Sci U S A. 2019. PMID: 31085645 Free PMC article. - What Is the "Relevant" Amyloid β42 Concentration?
Raskatov JA. Raskatov JA. Chembiochem. 2019 Jul 1;20(13):1725-1726. doi: 10.1002/cbic.201900097. Epub 2019 May 17. Chembiochem. 2019. PMID: 30835961 Free PMC article.
References
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. - PubMed
- Sengupta P, Roy S, Krueger JM. The ATP-cytokine-adenosine hypothesis: how the brain translates past activity into sleep. Sleep Biol Rhythms. 2011;9:29–33.
- Kattler H, Dijk DJ, Borbely A. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous