Selective BRAF inhibition decreases tumor-resident lymphocyte frequencies in a mouse model of human melanoma - PubMed (original) (raw)
Selective BRAF inhibition decreases tumor-resident lymphocyte frequencies in a mouse model of human melanoma
Anna Hooijkaas et al. Oncoimmunology. 2012.
Abstract
The development of targeted therapies and immunotherapies has markedly advanced the treatment of metastasized melanoma. While treatment with selective BRAF(V600E) inhibitors (like vemurafenib or dabrafenib) leads to high response rates but short response duration, CTLA-4 blocking therapies induce sustained responses, but only in a limited number of patients. The combination of these diametric treatment approaches may further improve survival, but pre-clinical data concerning this approach is limited. We investigated, using Tyr::CreER(T2)PTEN(F-/-)BRAF(F-V600E/+) inducible melanoma mice, whether BRAF(V600E) inhibition can synergize with anti-CTLA-4 mAb treatment, focusing on the interaction between the BRAF(V600E) inhibitor PLX4720 and the immune system. While PLX4720 treatment strongly decreased tumor growth, it did not induce cell death in BRAF(V600E)/PTEN(-/-) melanomas. More strikingly, PLX4720 treatment led to a decreased frequency of tumor-resident T cells, NK-cells, MDSCs and macrophages, which could not be restored by the addition of anti-CTLA-4 mAb. As this effect was not observed upon treatment of BRAF wild-type B16F10 tumors, we conclude that the decreased frequency of immune cells correlates to BRAF(V600E) inhibition in tumor cells and is not due to an off-target effect of PLX4720 on immune cells. Furthermore, anti-CTLA-4 mAb treatment of inducible melanoma mice treated with PLX4720 did not result in enhanced tumor control, while anti-CTLA-4 mAb treatment did improve the effect of tumor-vaccination in B16F10-inoculated mice. Our data suggest that vemurafenib may negatively affect the immune activity within the tumor. Therefore, the potential effect of targeted therapy on the tumor-microenvironment should be taken into consideration in the design of clinical trials combining targeted and immunotherapy.
Figures
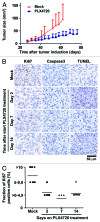
Figure 1. Selective BRAF inhibition in vivo results in strongly decreased tumor cell proliferation, but not induction of cell death, in BRAFV600E/PTEN−/− murine melanomas. (A) Four to ten weeks old Tyr::CreERT2PTENF−/−BRAFF-V600E/+ mice were induced on the flank and 31 d later, when average tumor size was 10 mm2, tumor-bearing mice were placed on PLX4720 or mock treatment. Tumor outgrowth was followed over time and tumor size in mm2 is plotted against time from tumor induction for PLX4720 treated (blue line, n = 10) and control chow treated animals (red line, n = 14). (B) Tumors were taken from Tyr::CreERT2PTENF−/−BRAFF-V600E/+ mice at distinct time points after start of PLX4720 treatment (day 0, 2, 7 and 14). Proliferation of tumor cells was measured by Ki67 immunohistochemistry. Tumor cell death was assessed by immunohistochemistry for active caspase-3 and by a TUNEL assay. Positive staining is displayed in red. (C) The percentage of Ki67-expressing tumor cells was scored into four categories and this quantification was graphically displayed (category 0 = 0%, category 1 ≥ 0–4,9%, category 2 = 5–9,9% and category 3 = 10% or more).
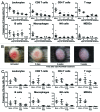
Figure 2. PLX4720 treatment leads to a decreased frequency of immune cells in BRAFV600E/PTEN−/− melanomas and this cannot be restored by CTLA-4 blockade. (A) Tumor-bearing Tyr::CreERT2PTENF−/−BRAFF-V600E/+ mice were mock or PLX4720 treated for 2, 7, 14 or 21 d. Tumors were removed directly following euthanasia and single cell suspensions were analyzed by use of flow cytometry. Dead cells were removed from all analyses (except for intracellular stainings) by discarding propidium iodide positive cells. Leukocytes were defined as being CD45+ cells. CD4+, CD8+ and regulatory T cells were respectively defined as the CD4+CD8-, CD4-CD8+ or the CD4+CD25+FoxP3+ population. B cells were characterized by their expression of B220 and CD19 while NK-cells were distinguished by the expression of NK1.1 in the absence of CD4 or CD8 expression. Myeoloid derived suppressor cells (MDSCs) and macrophages were respectively defined as the CD11b+GR1+ or CD11b+F4/80+ cell population. The shown values represent the frequency of the assessed cell population as a percentage of all living cells in the single cell suspension of the tumor for individually analyzed mice. As the T reg population was distinguished by use of an intracellular stain the values in this plot are shown as a percentage of all cells in the tumor suspension. (B) A tumor from a Tyr::CreERT2PTENF−/−BRAFF-V600E/+ mouse was placed on PLX4720 treatment and tumor appearance was followed photographically over time. Depicted is the tumor phenotype at start, day 5, day 14 and day 35 of PLX4720 treatment (C) Tyr::CreERT2PTENF−/−BRAFF-V600E/+ mice received a mock-treatment, PLX4720-treatment, anti-CTLA-4 mAb treatment (twice weekly for 6 weeks) or the combination of PLX4720 and CTLA-4 blockade treatment. The frequency of immune cells as a percentage of living cells in the tumor was assessed by flow cytometry as described for panel A.
Figure 3. Reduced tumor immune cell frequencies upon selective BRAF inhibition depends on the presence of the BRAFV600E mutation in tumor cells. (A) Tyr::CreERT2PTENF−/−BRAFF-V600E/+ mice received for at least 21 d a mock-treatment, PLX4720-treatment, anti-CTLA-4 mAb treatment (twice weekly for 6 weeks) or the combination of PLX4720 and CTLA-4 blockade treatment. Then the draining lymph node (dLN), contralateral lymph node (cLN) and spleen were removed directly following euthanasia and single cell suspensions were analyzed by use of flow cytometry as described for Figure 2A. (B) Nine week old male C57BL/6J mice were subcutaneously inoculated with 1x106 B16F10 cells in the shaven right flank. Four days after tumor inoculation mice were placed on PLX4720 or mock treatment and were subsequently sacrificed when tumors were at least 100 mm2 (500 mg) which was generally 10 to 21 d after tumor inoculation. The frequency of immune cells as a percentage of living cells in the tumor was assessed by flow cytometry as described for Figure 2A.
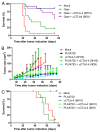
Figure 4. Addition of anti-CTLA-4 mAb treatment to PLX4720 treatment does not further improve tumor growth control, while it does when combined with Gvax-vaccination. (A) Nine week old female C57BL/6J mice were subcutaneously inoculated with 1x104 B16F10 cells in the shaven right flank at day 0. At day 0, 3 and 6 indicated mice received a subcutaneous injection in the contralateral flank with 150 Gray irradiated 1x106 GMCSF-expressing B16F10 cells (Gvax group, green line, n = 14) combined with intraperitoneal injections of 200 μg hamster-derived anti-CTLA-4 mAb clone 9H10 (blue line, n = 15) or 100 μg mouse-derived anti-CTLA-4 mAb clone 9D9 (purple line, n = 15). Tumor growth was followed over time by caliper measures and mice were sacrificed when tumors exceeded a 150 mm2 size or ulcerated. The survival of the different treatment groups was compared with that of mock treated mice (red line, n = 16) and depicted in a survival graph. (B) 4–10 week old Tyr::CreERT2PTENF−/−BRAFF-V600E/+ mice were induced on the flank and 31 d later, when average tumor size was 10 mm2, tumor-bearing mice were placed on mock treatment (red line, n = 11), PLX4720 treatment (dark blue line, n = 16), anti-CTLA-4 mAb clone 9H10 treatment (dark green line, n = 11) or PLX4720-treatment combined with either anti-CTLA-4 mAb clone 9H10 (light green line, n = 12) or clone 9D9 (light blue line, n = 8). Tumor growth was followed in two dimensions and graphed over time. (C) Survival of the mice described in Figure 4B was graphed over time.
Similar articles
- Natural killer cells are essential for the ability of BRAF inhibitors to control BRAFV600E-mutant metastatic melanoma.
Ferrari de Andrade L, Ngiow SF, Stannard K, Rusakiewicz S, Kalimutho M, Khanna KK, Tey SK, Takeda K, Zitvogel L, Martinet L, Smyth MJ. Ferrari de Andrade L, et al. Cancer Res. 2014 Dec 15;74(24):7298-308. doi: 10.1158/0008-5472.CAN-14-1339. Epub 2014 Oct 28. Cancer Res. 2014. PMID: 25351955 - Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma.
Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, Pinheiro EM, Koya RC, Graeber TG, Comin-Anduix B, Ribas A. Hu-Lieskovan S, et al. Sci Transl Med. 2015 Mar 18;7(279):279ra41. doi: 10.1126/scitranslmed.aaa4691. Sci Transl Med. 2015. PMID: 25787767 Free PMC article. - Targeting BRAFV600E in an inducible murine model of melanoma.
Hooijkaas AI, Gadiot J, van der Valk M, Mooi WJ, Blank CU. Hooijkaas AI, et al. Am J Pathol. 2012 Sep;181(3):785-94. doi: 10.1016/j.ajpath.2012.06.002. Epub 2012 Jul 11. Am J Pathol. 2012. PMID: 22796458 - Current and future roles of targeted therapy and immunotherapy in advanced melanoma.
Olszanski AJ. Olszanski AJ. J Manag Care Spec Pharm. 2014 Apr;20(4):346-56. doi: 10.18553/jmcp.2014.20.4.346. J Manag Care Spec Pharm. 2014. PMID: 24684639 Free PMC article. Review. - Targeting oncogenic Raf protein-serine/threonine kinases in human cancers.
Roskoski R Jr. Roskoski R Jr. Pharmacol Res. 2018 Sep;135:239-258. doi: 10.1016/j.phrs.2018.08.013. Epub 2018 Aug 15. Pharmacol Res. 2018. PMID: 30118796 Review.
Cited by
- Unsupervised Analysis Reveals the Involvement of Key Immune Response Genes and the Matrisome in Resistance to BRAF and MEK Inhibitors in Melanoma.
Liu-Smith F, Lin J. Liu-Smith F, et al. Cancers (Basel). 2024 Jun 24;16(13):2313. doi: 10.3390/cancers16132313. Cancers (Basel). 2024. PMID: 39001376 Free PMC article. - Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of US Food and Drug Administration-approved targeted therapy in advanced melanoma.
Kakadia S, Yarlagadda N, Awad R, Kundranda M, Niu J, Naraev B, Mina L, Dragovich T, Gimbel M, Mahmoud F. Kakadia S, et al. Onco Targets Ther. 2018 Oct 17;11:7095-7107. doi: 10.2147/OTT.S182721. eCollection 2018. Onco Targets Ther. 2018. PMID: 30410366 Free PMC article. Review. - The RhoJ-BAD signaling network: An Achilles' heel for BRAF mutant melanomas.
Ruiz R, Jahid S, Harris M, Marzese DM, Espitia F, Vasudeva P, Chen CF, de Feraudy S, Wu J, Gillen DL, Krasieva TB, Tromberg BJ, Pavan WJ, Hoon DS, Ganesan AK. Ruiz R, et al. PLoS Genet. 2017 Jul 28;13(7):e1006913. doi: 10.1371/journal.pgen.1006913. eCollection 2017 Jul. PLoS Genet. 2017. PMID: 28753606 Free PMC article. - Dissecting the Lymphatic System to Predict Melanoma Metastasis.
Suresh R, Ziemys A, Holder AM. Suresh R, et al. Front Oncol. 2020 Nov 27;10:576190. doi: 10.3389/fonc.2020.576190. eCollection 2020. Front Oncol. 2020. PMID: 33330052 Free PMC article. Review. - BRAF-inhibition and tumor immune suppression.
Steinberg SM, Turk MJ. Steinberg SM, et al. Oncoimmunology. 2015 Mar 6;4(2):e988039. doi: 10.4161/2162402X.2014.988039. eCollection 2015 Feb. Oncoimmunology. 2015. PMID: 25949884 Free PMC article. Review.
References
- Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–32. doi: 10.1084/jem.194.6.823. - DOI - PMC - PubMed
- van Elsas A, Sutmuller RP, Hurwitz AA, Ziskin J, Villasenor J, Medema JP, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:481–9. doi: 10.1084/jem.194.4.481. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials