Mechanisms of psychostimulant-induced structural plasticity - PubMed (original) (raw)
Mechanisms of psychostimulant-induced structural plasticity
Sam A Golden et al. Cold Spring Harb Perspect Med. 2012.
Abstract
Psychostimulants robustly induce alterations in neuronal structural plasticity throughout brain reward circuits. However, despite our extensive understanding of how these circuits modulate motivated behavior, it is still unclear whether structural plasticity within these regions drives pathological behavioral responses in addiction. Although these structural changes have been subjected to an exhaustive phenomenological characterization, we still have a limited understanding of the molecular mechanisms regulating their induction and the functional relevance of such changes in mediating addiction-like behavior. Here we have highlighted the known molecular pathways and intracellular signaling cascades that regulate psychostimulant-induced changes in neuronal morphology and synaptic restructuring, and we discuss them in the larger context of addiction behavior.
Figures
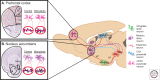
Figure 1.
Neural circuitry implicated in reward and underlying addiction. Several brain regions are directly involved in modulating reward and are associated with the pathology of substance abuse disorders. Dopaminergic VTA projection neurons (dotted purple lines) innervate the NAc and mPFC, as well as the hippocampus and amygdala. GABAergic afferents from the NAc (some direct and some indirect) (solid blue line) provide inhibitory feedback to dopaminergic VTA neurons. Excitory glutamatergic afferents (solid red lines) project to the NAc from the mPFC, hippocampus, and amygdala, as well as glutamatergic innervation of the VTA by the amygdala and hippocampus. Each region contains specialized cell types believed to play crucial roles in both natural reward phenomena and addiction-related phenotypes. These cell types, color-coded in the key, include amygdala (green) and NAc (purple) medium spiny neurons, mPFC (pink) and hippocampal CA3 (blue) pyramidal neurons, and VTA dopamine neurons (orange). Not shown are serotonergic projections from the DR and noradrenergic projections from the LC. Psychostimulants, as well as other drugs of abuse, robustly modulate the structural plasticity of individual neurons within these regions. Nissl-stained coronal sections of the (A) mPFC, and (B) NAc, with the left hemisphere of each marked schematically to represent subregions of interest. mPFC, Medial prefrontal cortex; NAc, nucleus accumbens; C-P, caudate-putamen; VP, ventral pallidum; LH, lateral hypothalamus; VTA, ventral tegmental area; SNr, substantia nigra; DR, dorsal raphe; LC, locus coeruleus; ACA, anterior cingulated; PL, prelimbic cortex; IL, infralimbic cortex; NAcc, nucleus accumbens core; NAcs, nucleus accumbens shell. (Adapted, with permission, from Russo et al. 2009.)
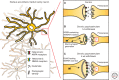
Figure 2.
Schematic of psychostimulant-induced synaptic and structural plasticity. (A) Repeated administration of psychostimulants results in rapid, although transient, shifts in α-amino-3-hydroxl-5-methyl-4-isoxazole-propionate (AMPA) glutamate receptor and _N_-methyl-
d
-aspartate (NMDA) receptor surface membrane expression in the postsynaptic density (PSD) of nucleus accumbens (NAc) medium spiny neurons (MSNs). These shifts in receptor composition are coupled to alterations in dendritic spine structural plasticity, which correlate with types of synaptic plasticity. (B) Specifically, acute withdrawal from chronic cocaine induces the formation of thin spines and silent synapses via insertion of NR2B-containing NMDARs, as well as results in long-term depression (LTD). (C) As the withdrawal period extends, GluA2-lacking AMPARs are inserted into the spine head, and there is a shift toward mushroom spines with increased spine head diameter and enhanced long-term potentiation (LTP). Acute reexposure to cocaine following extended withdrawal periods (not depicted above) results in a rapid induction of thin spines and LTD, similar to what is observed in panel B. The role of presynaptic structural plasticity is currently unknown.
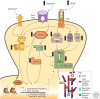
Figure 3.
Molecular mechanisms and signaling pathways implicated in psychostimulant-induced structural plasticity. Several transcription factors, including, but not limited to, NFκB, ΔFosB, myocyte enhancing factor 2 (MEF2), and cyclic AMP response element binding protein (CREB), regulate dendritic spine structural and synaptic plasticity. In addition to dopamine and opioid neurotransmission, brain-derived neurotrophic factor (BDNF) and other neurotrophic signals regulate this transcriptional machinery via receptor tyrosine kinase-modulated activation of phosphoinositide 3-kinase (PI3K), thymoma viral proto-oncogene (Akt), Ras-extracellular regulated kinase (ERK), and NFκB pathways. The common downstream effectors of these signaling cascades are believed to be, in large part, members of the small RhoGTPase family such as Ras-related C3 botulinum toxin substrate 1 (Rac1), which then alter actin cytoskeletal dynamics through downstream targeting of PAK, LIMK, and ultimately cofilin. It is further speculated that NFκB activation may occur through a cytokine receptor–dependent mechanism to control spine plasticity; however, this is yet to be proven empirically. Psychostimulant-induced changes in structural plasticity therefore have many potential signaling mechanisms through which to alter behavioral and molecular effects, often culminating in modulation of actin assembly and cycling via altered gene expression. PLCγ, phospholipase Cγ; IκK, inhibitor of κB kinase; IκB, inhibitor of κB; TrkB, tyrosine receptor kinase B; Drd, dopamine receptor; LIMK, lim domain kinase; WASP, Wiskott-Aldrich Syndrome proteins; Cdk5, cyclin-dependent kinase-5.
Similar articles
- Molecular mechanisms of psychostimulant-induced structural plasticity.
Dietz DM, Dietz KC, Nestler EJ, Russo SJ. Dietz DM, et al. Pharmacopsychiatry. 2009 May;42 Suppl 1(Suppl 1):S69-78. doi: 10.1055/s-0029-1202847. Epub 2009 May 11. Pharmacopsychiatry. 2009. PMID: 19434558 Free PMC article. Review. - Structural plasticity of the brain to psychostimulant use.
Nyberg F. Nyberg F. Neuropharmacology. 2014 Dec;87:115-24. doi: 10.1016/j.neuropharm.2014.07.004. Epub 2014 Jul 11. Neuropharmacology. 2014. PMID: 25018041 Review. - Histone Deacetylases and Immediate Early Genes: Key Players in Psychostimulant-Induced Neuronal Plasticity.
Bisagno V, Cadet JL. Bisagno V, et al. Neurotox Res. 2021 Dec;39(6):2134-2140. doi: 10.1007/s12640-021-00420-3. Epub 2021 Sep 28. Neurotox Res. 2021. PMID: 34581974 Review. - Glycogen synthase kinase-3 signaling in cellular and behavioral responses to psychostimulant drugs.
Barr JL, Unterwald EM. Barr JL, et al. Biochim Biophys Acta Mol Cell Res. 2020 Sep;1867(9):118746. doi: 10.1016/j.bbamcr.2020.118746. Epub 2020 May 23. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 32454064 Free PMC article. Review. - Opioid and Psychostimulant Plasticity: Targeting Overlap in Nucleus Accumbens Glutamate Signaling.
Hearing M, Graziane N, Dong Y, Thomas MJ. Hearing M, et al. Trends Pharmacol Sci. 2018 Mar;39(3):276-294. doi: 10.1016/j.tips.2017.12.004. Epub 2018 Jan 12. Trends Pharmacol Sci. 2018. PMID: 29338873 Free PMC article. Review.
Cited by
- Psychostimulant Use Disorder, an Unmet Therapeutic Goal: Can Modafinil Narrow the Gap?
Hersey M, Bacon AK, Bailey LG, Coggiano MA, Newman AH, Leggio L, Tanda G. Hersey M, et al. Front Neurosci. 2021 May 26;15:656475. doi: 10.3389/fnins.2021.656475. eCollection 2021. Front Neurosci. 2021. PMID: 34121988 Free PMC article. Review. - Targeting the cytoskeleton as a therapeutic approach to substance use disorders.
Pandey S, Miller CA. Pandey S, et al. Pharmacol Res. 2024 Apr;202:107143. doi: 10.1016/j.phrs.2024.107143. Epub 2024 Mar 16. Pharmacol Res. 2024. PMID: 38499081 Free PMC article. Review. - Cell-Type-Specific Adaptions in Striatal Medium-Sized Spiny Neurons and Their Roles in Behavioral Responses to Drugs of Abuse.
Allichon MC, Ortiz V, Pousinha P, Andrianarivelo A, Petitbon A, Heck N, Trifilieff P, Barik J, Vanhoutte P. Allichon MC, et al. Front Synaptic Neurosci. 2021 Dec 14;13:799274. doi: 10.3389/fnsyn.2021.799274. eCollection 2021. Front Synaptic Neurosci. 2021. PMID: 34970134 Free PMC article. Review. - Transforming growth factor beta receptor 1 is increased following abstinence from cocaine self-administration, but not cocaine sensitization.
Gancarz-Kausch AM, Schroeder GL, Panganiban C, Adank D, Humby MS, Kausch MA, Clark SD, Dietz DM. Gancarz-Kausch AM, et al. PLoS One. 2013 Dec 30;8(12):e83834. doi: 10.1371/journal.pone.0083834. eCollection 2013. PLoS One. 2013. PMID: 24386286 Free PMC article. - Kalirin-7 mediates cocaine-induced AMPA receptor and spine plasticity, enabling incentive sensitization.
Wang X, Cahill ME, Werner CT, Christoffel DJ, Golden SA, Xie Z, Loweth JA, Marinelli M, Russo SJ, Penzes P, Wolf ME. Wang X, et al. J Neurosci. 2013 Jul 3;33(27):11012-22. doi: 10.1523/JNEUROSCI.1097-13.2013. J Neurosci. 2013. PMID: 23825406 Free PMC article.
References
- Abraham WC, Bear MF 1996. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci 19: 126–130 - PubMed
- Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE 1999. Environmental modulation of amphetamine-induced c-fos expression in D1 versus D2 striatal neurons. Behav Brain Res 103: 203–209 - PubMed
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW 2003. Neuroadaptations in cystine–glutamate exchange underlie cocaine relapse. Nat Neurosci 6: 743–749 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources