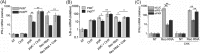Protein kinase PKR amplification of interferon β induction occurs through initiation factor eIF-2α-mediated translational control - PubMed (original) (raw)
Protein kinase PKR amplification of interferon β induction occurs through initiation factor eIF-2α-mediated translational control
Christopher S McAllister et al. J Biol Chem. 2012.
Abstract
The protein kinase PKR is activated by RNA with double-stranded (ds) structure and subsequently impairs translation through phosphorylation of protein synthesis initiation factor eIF-2α. PKR also mediates activation of signal transduction pathways leading to interferon beta (IFN-β) gene induction following virus-infection or RNA transfection. We previously demonstrated in measles virus-infected cells that PKR is required for the maximal induction of IFN-β gene expression by the interferon promoter stimulator gene 1 (IPS-1) adaptor-dependent cytosolic RNA sensor pathway. While both IPS-1 and PKR are important mediators of IFN-β induction, with PKR contributing to an enhanced NF-κB activation, the mechanism by which PKR enhances NF-κB activity and amplifies IFN-β induction is unresolved. Herein we tested the possibility that PKR could activate signal transduction pathways indirectly through translational control responses. Following transfection with synthetic or natural dsRNAs or infection with measles virus, we observed increased mRNA but decreased protein levels for the inhibitor of NF-κB signaling, IκB-α, that correlated with PKR activation and eIF-2α phosphorylation. Importantly, knockdown of PKR increased IκB-α protein levels and impaired IFN-β induction. Additionally, inhibition of translation by cycloheximide treatment rescued IFN-β induction following PKR knockdown but not IPS-1 knockdown. Mutation of eIF-2α to prevent phosphorylation also impaired IFN-β induction in PKR-sufficient virus-infected cells. These results suggest that an eIF-2α-dependent translation inhibition mechanism is sufficient to explain the PKR-mediated amplification of IPS-1-dependent IFN-β induction by foreign RNA.
Figures
FIGURE 1.
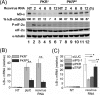
dsRNA length-dependent induction of IFN-β correlates with PKR-dependent reduction in IκB-α protein and eIF-2α phosphorylation. A, relative IFN-β induction measured by quantitative PCR (qPCR) using RNA extracted 5 h following incubation with lipid alone (NT) or RNA transfection in parental PKR+ HeLa cells. IFN-β mRNA levels were normalized to GAPDH using the ΔΔCT method. Standard deviation determined from three independent experiments. B, Western blot for IκB-α, phosphorylated eIF-2α (P-eIF-2α) and total eIF-2α, and α-tubulin loading control, at 4 h and 8 h following transfection of either parental PKR+ or knockdown PKRkd HeLa cells with the indicated dsRNA. C, quantitation of IκB-α protein level normalized to α-tubulin and compared with cells not transfected (NT). Mean and standard deviation determined from two independent experiments. *, p < 0.05 compared with NT and Pds20 at the corresponding time point in PKR+ cells, **, p < 0.01 compared with NT and Pds20.
FIGURE 2.
PKR-dependent reduction in IκB-α protein level inversely correlates with IκB-α mRNA level following MV infection. A, Western blot for IκB-α protein following mock, WT, Vko, and Cko measles virus infection of PKR+ and PKRkd cells. IκB-α protein levels normalized to α-tubulin in each lane are expressed as a percentage of the mock infected lane. B, IκB-α mRNA transcript levels measured by qPCR at 24 h postinfection in PKR+ and PKRkd cells as described in Fig. 1. Mean and standard deviation are shown from two independent experiments analyzed in triplicate. *, p < 0.05 compared with mock treatment in PKR+ cells, **, p < 0.01.
FIGURE 3.
PKR-dependent reduction in IκB-α protein level and increase in IκB-α mRNA level following transfection with reovirus genome dsRNA. A, Western blot for IκB-α, phosphorylated eIF-2α (P-eIF-2α) and total eIF-2α, and α-tubulin loading control, at the indicated times after transfection of PKR+ or PKRkd cells with purified reovirus genome dsRNA. Percentage (%) indicates IκB-α protein level normalized to α-tubulin and compared with cells not transfected. B, IκB-α mRNA transcript level measured by qPCR with RNA isolated from PKR+ and PKRkd cells at 5 h following transfection with reovirus genome dsRNA or pIpC. Error bars represent the standard deviation determined from three independent experiments. C, qPCR measurement of IκB-α mRNA transcript level following transfection with reovirus genome dsRNA of HeLa cells transiently knocked down for PKR, IPS-1 or TRIF or transfected with a control siRNA (siLUC). Standard deviations determined from two independent experiments analyzed in triplicate. *, p < 0.05; **, p < 0.01.
FIGURE 4.
**Translation inhibition by cycloheximide prevents IκB-**α protein expression in PKRkd cells. PKR+ and PKRkd cells were transfected with reovirus genome dsRNA for the indicated period of time (h) or not transfected (NT), and were treated (+) with cycloheximide (CHX) or left untreated (−) as indicated. Western immunoblot analyses were performed for IκB-α, phosphorylated eIF-2α (P-eIF-2α), total eIF-2α, phosphorylated PKR (P-PKR), and total PKR, and α-tubulin as a loading control. Percentage (%) is calculated for IκB-α protein level normalized to α-tubulin, and compared with PKR+ cells not treated with CHX and not transfected. Extracts from PKRkd cells, transfected with reovirus genome dsRNA but not treated with CHX, are shown in lanes 13 and 14 for comparison.
FIGURE 5.
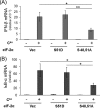
**Translation inhibition by cycloheximide rescues PKR-dependent but not IPS-1-dependent induction of IFN-**β transcripts. A, RNA was isolated from PKR+ or PKRkd cells at 5 h after transfection with pIpC or reovirus genome dsRNA in the presence or absence of CHX as indicated and the relative IFN-β mRNA transcript level was determined by qPCR as described in Fig. 1. The standard deviation was determined from two or three independent experiments. B, qPCR measurement of relative IκB-α mRNA transcript levels following treatment conditions described in A. C, relative IFN-β mRNA transcript level in parental PKR+ HeLa cells transiently knocked down for either PKR or IPS-1, or transfected with a control siLUC siRNA prior to transfection of reovirus genome dsRNA in the absence or presence of CHX. Standard deviation is shown for two independent experiments. *, p < 0.05; **, p < 0.01.
FIGURE 6.
Expression of mutant eIF-2α (S48,51A) in PKR+ cells decreases IFN-β induction by measles virus. Cells (PKR+) were transfected with either an empty vector (Vec) or an expression construct encoding human eIF-2α mutated to prevent phosphorylation (S48,51A) or a phospho-mimetic (S51D) mutant. At 20 h after transfection, cells were either left uninfected (−) or infected (+) with MV Cko virus, and at 24 h after infection total RNA was prepared and transcript levels determined by qPCR. The results shown are normalized to GAPDH transcript level and are relative to uninfected, vector-transfected cells. The mean induction and standard deviation were determined from three independent experiments. *, p < 0.05; **, p < 0.01. A, IFN-β transcript levels; B, IκB-α transcript levels.
FIGURE 7.
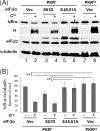
Expression of mutant eIF-2α (S48,51A) increases IκB-α protein levels in Cko measles virus infected PKR+ cells to levels seen in PKRkd-infected cells. A, PKR+ or PKRkd cells were transfected with either an empty vector (Vec) or an expression construct encoding either S48,51A or S51D mutant eIF-2α as indicated. At 20 h after transfection cells were either left uninfected (−) or infected (+) with MV Cko virus, and at 24 h after infection extracts were prepared and western immunoblot analyses performed using antibodies against IκB-α, MV H protein and eIF-2α, and α-tubulin as a loading control. B, quantitation of IκB-α protein levels normalized to α-tubulin; average and standard deviation determined from three independent analyses; **, p < 0.01.
Similar articles
- Mechanisms of protein kinase PKR-mediated amplification of beta interferon induction by C protein-deficient measles virus.
McAllister CS, Toth AM, Zhang P, Devaux P, Cattaneo R, Samuel CE. McAllister CS, et al. J Virol. 2010 Jan;84(1):380-6. doi: 10.1128/JVI.02630-08. J Virol. 2010. PMID: 19846517 Free PMC article. - Regulation of mRNA translation and cellular signaling by hepatitis C virus nonstructural protein NS5A.
He Y, Tan SL, Tareen SU, Vijaysri S, Langland JO, Jacobs BL, Katze MG. He Y, et al. J Virol. 2001 Jun;75(11):5090-8. doi: 10.1128/JVI.75.11.5090-5098.2001. J Virol. 2001. PMID: 11333890 Free PMC article. - Protein kinase PKR and RNA adenosine deaminase ADAR1: new roles for old players as modulators of the interferon response.
Pfaller CK, Li Z, George CX, Samuel CE. Pfaller CK, et al. Curr Opin Immunol. 2011 Oct;23(5):573-82. doi: 10.1016/j.coi.2011.08.009. Epub 2011 Sep 15. Curr Opin Immunol. 2011. PMID: 21924887 Free PMC article. Review. - Old target new approach: an alternate NF-kappaB activation pathway via translation inhibition.
László CF, Wu S. László CF, et al. Mol Cell Biochem. 2009 Aug;328(1-2):9-16. doi: 10.1007/s11010-009-0067-8. Epub 2009 Feb 18. Mol Cell Biochem. 2009. PMID: 19224334 Free PMC article. Review.
Cited by
- The monkeypox virus-host interplays.
Yi XM, Lei YL, Li M, Zhong L, Li S. Yi XM, et al. Cell Insight. 2024 Jul 14;3(5):100185. doi: 10.1016/j.cellin.2024.100185. eCollection 2024 Oct. Cell Insight. 2024. PMID: 39144256 Free PMC article. Review. - Crosstalk between vault RNAs and innate immunity.
Avila-Bonilla RG, Martínez-Montero JP. Avila-Bonilla RG, et al. Mol Biol Rep. 2024 Mar 5;51(1):387. doi: 10.1007/s11033-024-09305-y. Mol Biol Rep. 2024. PMID: 38443657 Free PMC article. Review. - Nucleic acid-induced inflammation on hematopoietic stem cells.
Vu GT, Awad V, Norberto MF, Bowman TV, Trompouki E. Vu GT, et al. Exp Hematol. 2024 Mar;131:104148. doi: 10.1016/j.exphem.2023.104148. Epub 2023 Dec 25. Exp Hematol. 2024. PMID: 38151171 - Loss of ADAR1 in macrophages in combination with interferon gamma suppresses tumor growth by remodeling the tumor microenvironment.
Lin W, Luo Y, Wu J, Zhang H, Jin G, Guo C, Zhou H, Liang H, Xu X. Lin W, et al. J Immunother Cancer. 2023 Nov;11(11):e007402. doi: 10.1136/jitc-2023-007402. J Immunother Cancer. 2023. PMID: 37935565 Free PMC article. - Immunomodulatory Role of Interferons in Viral and Bacterial Infections.
Mertowska P, Smolak K, Mertowski S, Grywalska E. Mertowska P, et al. Int J Mol Sci. 2023 Jun 14;24(12):10115. doi: 10.3390/ijms241210115. Int J Mol Sci. 2023. PMID: 37373262 Free PMC article. Review.
References
- Akira S., Takeda K. (2004) Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 - PubMed
- Meylan E., Tschopp J. (2006) Toll-like receptors and RNA helicases: Two parallel ways to trigger antiviral responses. Mol. Cell 22, 561–569 - PubMed
- Kawai T., Takahashi K., Sato S., Coban C., Kumar H., Kato H., Ishii K. J., Takeuchi O., Akira S. (2005) IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6, 981–988 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI-12520/AI/NIAID NIH HHS/United States
- R01 AI020611/AI/NIAID NIH HHS/United States
- R37 AI012520/AI/NIAID NIH HHS/United States
- R01 AI012520/AI/NIAID NIH HHS/United States
- AI-20611/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous