TRIM56 is an essential component of the TLR3 antiviral signaling pathway - PubMed (original) (raw)
TRIM56 is an essential component of the TLR3 antiviral signaling pathway
Yang Shen et al. J Biol Chem. 2012.
Abstract
Members of the tripartite motif (TRIM) proteins are being recognized as important regulators of host innate immunity. However, specific TRIMs that contribute to TLR3-mediated antiviral defense have not been identified. We show here that TRIM56 is a positive regulator of TLR3 signaling. Overexpression of TRIM56 substantially potentiated extracellular dsRNA-induced expression of interferon (IFN)-β and interferon-stimulated genes (ISGs), while knockdown of TRIM56 greatly impaired activation of IRF3, induction of IFN-β and ISGs, and establishment of an antiviral state by TLR3 ligand and severely compromised TLR3-mediated chemokine induction following infection by hepatitis C virus. The ability to promote TLR3 signaling was independent of the E3 ubiquitin ligase activity of TRIM56. Rather, it correlated with a physical interaction between TRIM56 and TRIF. Deletion of the C-terminal portion of TRIM56 abrogated the TRIM56-TRIF interaction as well as the augmentation of TLR3-mediated IFN response. Together, our data demonstrate TRIM56 is an essential component of the TLR3 antiviral signaling pathway and reveal a novel role for TRIM56 in innate antiviral immunity.
Figures
FIGURE 1.
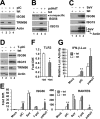
Effects of tet-inducible expression of TRIM56 on activation of the IFN response via different signaling pathways. HeLa-FitA2-T56 cells with tet-regulated conditional expression of WT HA-TRIM56 were manipulated to repress (−tet) or induce (+tet) HA-TRIM56 expression, followed by stimulation by extracellular poly-I:C (pIC, panel A), transfected poly-dA:dT (pdAdT, panel B), SeV infection (panel C), and transfected poly-I:C (T-pIC, panel D) for 12 h. Cell lysates were harvested for immunoblot analysis of ISG56, ISG15, TRIM56, and actin expression. In panel B, a nonspecific band (marked by filled circle) detected by the anti-ISG56 antibody served as a loading control. Two different exposures (short- and long-exposure) of the ISG15 blot are shown. In panel C, the blot at the bottom was detected by a mixture of anti-SeV and anti-actin antibodies. The anti-SeV antibody (Keskinen et al., Ref. 43) mainly reacted with two viral proteins, i.e. hemagglutinin-neuraminidase and nucleocapsid, with the latter having a molecular mass similar to actin. Note that in panels A and D, the anti-TRIM56 antibody detected only the endogenous TRIM56 protein in cells cultured in the absence of tet (lanes 2 and 4), which migrated slightly faster than the HA-TRIM56 detected in induced cells (+tet, lanes 1 and 3). E, HeLa-FitA2-T56 cells were manipulated and treated similarly to those in panels A–D, but cells were harvested for RNA extraction and qPCR analysis of ISG56 and RANTES mRNA expression. * and ** indicate statistical differences exist between −tet and +tet cells with a p value of <0.05 and <0.01, respectively. F, qPCR analysis of TLR3 mRNA levels in HeLa-FitA2-T56 cells repressed and induced for HA-TRIM56 expression. G, reporter gene assay of pIC- or pdAdT-induced IFN-β promoter activities in HeLa-FitA2-T56 cells without (−tet) or with (+tet) HA-TRIM56 expression.
FIGURE 2.
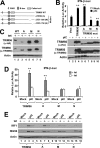
The C-terminal portion, but not the RING domain, controls the ability of TRIM56 to promote TLR3 signaling. A, schematic representation of the domain structures of WT TRIM56 and the individual TRIM56 deletion mutants. B, upper panel: activation of the IFN-β promoter by extracellular poly-I:C in HEK293-TLR3 cells transiently transfected with vectors encoding WT or the indicated mutant TRIM56. Vect: empty vector. * and ** indicate statistical differences exist with a p value of <0.05 and <0.01, respectively, when compared with cells transfected with empty vector and stimulated by poly-I:C. Expression of WT/mutant TRIM56 proteins in cell lysates was analyzed by immunoblotting using either anti-HA or anti-TRIM56 antibody and shown in lower panel. Note that the A mutant was expressed at a level similar to that of WT or the M or N mutant TRIM56 when detected by anti-TRIM56 antibody. The lower expression level of this mutant revealed by immunoblotting with anti-HA antibody likely reflects the different folding of this mutant from WT and other mutant forms of TRIM56 that partially masks the HA epitope introduced to the N terminus of the protein. C, immunoblot analysis of TRIM56 and actin expression in HeLa-FitA2-derived cell lines conditionally expressing WT or the indicated TRIM56 mutant. Note that the exogenous introduced HA-TRIM56 (WT or mutants) could be detected in +tet cells by either anti-TRIM56 or anti-HA tag antibodies. D, activation of the IFN-β promoter by extracellular poly-I:C in HeLa-FitA2-derived cell lines conditionally expressing WT or the indicated TRIM56 mutant. ** indicates statistical difference exists between −tet and +tet cells with a p value of <0.01. E, immunoblotting of IRF1, ISG56 and actin expression in HeLa-FitA2-derived cell lines conditionally expressing WT or the indicated TRIM56 mutant following mock-stimulation or stimulation by extracellular poly-I:C for 12 h.
FIGURE 3.
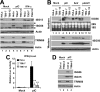
Effects of TRIM56 knockdown on IFN induction and ISG expression in HeLa cells following various stimuli. A, control HeLa and HeLa cells with stable knockdown of TRIM56 (T56i-7 and T56i-12) were mock-treated, stimulated with poly-I:C or IFN-α for 12 h. Cells were lysed for immunoblotting of ISG15, ISG56, TRIM56 (two different exposures of the blot are shown) and actin expression. Filled circles denote nonspecific bands. B, control HeLa, HeLa T56i-7 and T56i-12 cells were mock-treated, stimulated with poly-I:C, infected with SeV, or transfected with poly-dA:dT for 12 h. Northern blotting was performed to quantify the expression of ISG56 transcript. The ethidium bromide-stained 18S and 28S rRNAs served as loading controls (upper panel). Western blotting (lower panel) was carried out to determine the expression levels of ISG56 protein (two different exposures of the blot are shown). Actin was blotted to demonstrate equal sample loading. C, IFN-β promoter activities in control HeLa, HeLa T56i-7, and T56i-12 cells mock-stimulated or stimulated by poly-I:C for 8 h. ** indicates statistical difference exists with a p value of <0.01 when compared with HeLa cells stimulated by poly-I:C. D, HeLa cells were transiently transfected with a scrambled control siRNA or TRIM56 siRNA#2. 48 h later, cells were mock-treated or stimulated by poly-I:C for 12 h, followed by cell lysis and immunoblotting of ISG56, ISG15, TRIM56, and actin expression. Note that poly-I:C moderately up-regulated TRIM56 expression in cells transfected with control siRNA (compare lanes 3 versus 1).
FIGURE 4.
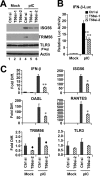
Knockdown of TRIM56 impairs induction of type I IFN response by poly-I:C in HEK293-TLR3 cells. HEK293-TLR3 cells were transiently transfected with control siRNA or different TRIM56 siRNAs for 48 h, followed by mock-stimulation or stimulation by poly-I:C for 12 h (panel A) or 6 h (panel C). Cells were then harvested for immunoblotting of ISG56, TRIM56, TLR3 (using anti-Flag antibody) and actin (panel A) or for qPCR analysis of the abundance of IFN-β, ISG56, OASL, RANTES, TRIM56, and TLR3 mRNAs (panel C). Note that poly-I:C moderately up-regulated TRIM56 expression in cells transfected with control siRNA (compare lanes 4 versus 1 in panel A). In panel C, * and ** indicate statistical differences exist with a p value of <0.05 and <0.01, respectively, when compared with cells transfected with control siRNA and stimulated by poly-I:C. ♦ denotes p = 0.05∼0.06 between cells transfected with control siRNA and TRIM56 siRNA-2. B, poly-I:C (12 h)-induced IFN-β promoter activation in HEK293-TLR3 cells transiently transfected with control siRNA or different TRIM56 siRNAs. ** indicates statistical difference exists with a p value of <0.01 when compared with cells transfected with control siRNA and stimulated by poly-I:C.
FIGURE 5.
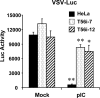
TRIM56 depletion compromises the establishment of an antiviral state by TLR3 ligand. Control HeLa, HeLa T56i-7, and T56i-12 cells were mock-stimulated or stimulated by poly-I:C, followed by infection with VSV-Luc (MOI = 0.1). At 3 h postinfection, cells were lysed for firefly luciferase assay. * and ** indicate statistical differences exist between mock-treated and poly-I:C-stimulated cells with a p value of <0.05 and <0.01, respectively.
FIGURE 6.
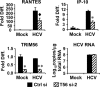
Knockdown of TRIM56 dampens TLR3-mediated chemokine induction by HCV infection in Huh7.5-TLR3 cells. Huh7.5-TLR3 cells were transiently transfected with control siRNA or TRIM56 siRNA#2. 30 h later, cells were mock-infected or infected by HCV (MOI = 0.5). At 48 h postinfection, cells were lysed for total RNA isolation, followed by quantification of RANTES, IP-10, and TRIM56 mRNAs and HCV RNA levels by qPCR. * and ** indicate statistical differences exist with a p value of <0.05 and <0.01, respectively, when compared with cells transfected with control siRNA. ♦ denotes p = 0.06 between cells transfected with control siRNA and TRIM56 siRNA-2.
FIGURE 7.
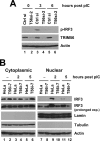
Silencing the expression of TRIM56 diminishes poly-I:C-induced IRF3 phosphorylation and nuclear translocation. A, HeLa cells were transiently transfected with control siRNA or TRIM56 siRNA#2 for 48 h, followed by stimulation by poly-I:C for the indicated time periods. Whole cell lysates were subjected to immunoblot analysis of phosphorylated IRF3 (using anti-IRF3 p396 antibody), TRIM56, and actin. B, control HeLa and HeLa T56i-7 cells were stimulated by poly-I:C for the indicated time periods. Cytoplasmic and nuclear extracts were isolated and subjected to immunoblotting of IRF3, Lamin A/C (a nuclear protein marker), tubulin (a cytoplasmic protein marker), and actin. Comparison of the two different exposures of the IRF3 blot showed that the IRF3 protein present in poly-I:C-stimulated HeLa nuclear extracts was phosphorylated IRF3.
FIGURE 8.
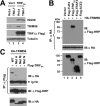
TRIM56 forms a complex with TRIF. A, immunoblot analysis of ISG56, TRIM56, TRIF (using anti-Flag antibody), and actin in control HeLa and HeLa T56i-7 cells transfected with an empty vector (lanes 1–2) or an vector encoding Flag-TRIFSA (lanes 3–4)for 24 h. B, co-IP experiment in HEK293 cells co-expressing HA-TRIM56 (WT) and various Flag-tagged signaling proteins. Cells co-expressing Flag-ACE2 (lane 5) or empty vector (lane 1) served as negative controls. Cell lysates were immunoprecipitated (IP) with anti-HA, followed by immunoblotting (IB) with anti-Flag-HRP or anti-HA. The bottom blot showed expression of the various Flag-tagged proteins in cell lysates. C, co-IP experiment in HEK293 cells co-expressing Flag- TRIFSA and WT HA-TRIM56 or various mutant HA-TRIM56. Cell lysates were immunoprecipitated (IP) with anti-Flag, followed by immunoblotting (IB) with anti-HA or anti-Flag-HRP. The bottom blot showed expression of the various HA-TRIM56 proteins (WT or mutant) in cell lysates. Ig Hc: IgG heavy chain.
Similar articles
- Key roles for phosphorylation and the Coiled-coil domain in TRIM56-mediated positive regulation of TLR3-TRIF-dependent innate immunity.
Liu BM, Li NL, Wang R, Li X, Li ZA, Marion TN, Li K. Liu BM, et al. J Biol Chem. 2024 May;300(5):107249. doi: 10.1016/j.jbc.2024.107249. Epub 2024 Mar 29. J Biol Chem. 2024. PMID: 38556084 Free PMC article. - TRIM56 Suppresses Multiple Myeloma Progression by Activating TLR3/TRIF Signaling.
Chen Y, Zhao J, Li D, Hao J, He P, Wang H, Zhang M. Chen Y, et al. Yonsei Med J. 2018 Jan;59(1):43-50. doi: 10.3349/ymj.2018.59.1.43. Yonsei Med J. 2018. PMID: 29214775 Free PMC article. - Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses.
Chen Y, Lin J, Zhao Y, Ma X, Yi H. Chen Y, et al. J Zhejiang Univ Sci B. 2021 Aug 15;22(8):609-632. doi: 10.1631/jzus.B2000808. J Zhejiang Univ Sci B. 2021. PMID: 34414698 Free PMC article. Review. - Functional evolution of the TICAM-1 pathway for extrinsic RNA sensing.
Seya T, Matsumoto M, Ebihara T, Oshiumi H. Seya T, et al. Immunol Rev. 2009 Jan;227(1):44-53. doi: 10.1111/j.1600-065X.2008.00723.x. Immunol Rev. 2009. PMID: 19120474 Review.
Cited by
- The implication of infection with respiratory syncytial virus in pediatric recurrent wheezing and asthma: knowledge expanded post-COVID-19 era.
Su P, Jiang C, Zhang Y. Su P, et al. Eur J Clin Microbiol Infect Dis. 2024 Mar;43(3):403-416. doi: 10.1007/s10096-023-04744-0. Epub 2023 Dec 28. Eur J Clin Microbiol Infect Dis. 2024. PMID: 38153660 Review. - Roles of long non-coding RNAs and emerging RNA-binding proteins in innate antiviral responses.
Wang Y, Wang Y, Luo W, Song X, Huang L, Xiao J, Jin F, Ren Z, Wang Y. Wang Y, et al. Theranostics. 2020 Jul 23;10(20):9407-9424. doi: 10.7150/thno.48520. eCollection 2020. Theranostics. 2020. PMID: 32802200 Free PMC article. Review. - Adult-Onset Still's Disease (AOSD)-On the Basis of Own Cases.
Wisłowska M. Wisłowska M. Biomedicines. 2024 Sep 10;12(9):2067. doi: 10.3390/biomedicines12092067. Biomedicines. 2024. PMID: 39335580 Free PMC article. - Pulmonary microbial spectrum in late-stage SARS-CoV-2 infection: a case series.
Hong JJ, Zhang RT, Ma CL, Hu QY. Hong JJ, et al. Eur J Clin Microbiol Infect Dis. 2024 Oct;43(10):2037-2046. doi: 10.1007/s10096-024-04897-6. Epub 2024 Jul 20. Eur J Clin Microbiol Infect Dis. 2024. PMID: 39031269 - TRIM56-mediated monoubiquitination of cGAS for cytosolic DNA sensing.
Seo GJ, Kim C, Shin WJ, Sklan EH, Eoh H, Jung JU. Seo GJ, et al. Nat Commun. 2018 Feb 9;9(1):613. doi: 10.1038/s41467-018-02936-3. Nat Commun. 2018. PMID: 29426904 Free PMC article.
References
- Sen G. C. (2001) Viruses and interferons. Annu. Rev. Microbiol. 55, 255–281 - PubMed
- Kawai T., Akira S. (2008) Toll-like receptor and RIG-I-like receptor signaling. Ann. N.Y. Acad. Sci. 1143, 1–20 - PubMed
- Yoneyama M., Fujita T. (2010) Recognition of viral nucleic acids in innate immunity. Rev. Med. Virol. 20, 4–22 - PubMed
- Seth R. B., Sun L., Ea C. K., Chen Z. J. (2005) Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell 122, 669–682 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases