Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation - PubMed (original) (raw)
Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation
Christine Juliana et al. J Biol Chem. 2012.
Abstract
The NLRP3 inflammasome is a key component of the innate immune response to pathogenic infection and tissue damage. It is also involved in the pathogenesis of a number of human diseases, including gouty arthritis, silicosis, atherosclerosis, and type 2 diabetes. The assembly of the NLRP3 inflammasome requires a priming signal derived from pattern recognition or cytokine receptors, followed by a second signal derived from extracellular ATP, pore-forming toxins, or crystalline materials. How these two signals activate the NLRP3 inflammasome is not yet clear. Here, we show that in mouse macrophages, signaling by the pattern recognition receptor TLR4 through MyD88 can rapidly and non-transcriptionally prime NLRP3 by stimulating its deubiquitination. This process is dependent on mitochondrial reactive oxygen species production and can be inhibited by antioxidants. We further show that signaling by ATP can also induce deubiquitination of NLRP3 by a mechanism that is not sensitive to antioxidants. Pharmacological inhibition of NLRP3 deubiquitination completely blocked NLRP3 activation in both mouse and human cells, indicating that deubiquitination of NLRP3 is required for its activation. Our findings suggest that NLRP3 is activated by a two-step deubiquitination mechanism initiated by Toll-like receptor signaling and mitochondrial reactive oxygen species and further potentiated by ATP, which could explain how NLRP3 is activated by diverse danger signals.
Figures
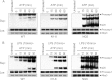
FIGURE 1.
LPS-induced priming of the NLRP3 inflammasome does not require new protein synthesis. A and C, immunoblots of caspase-1 in the culture supernatants (Sup; upper panels) or cell lysates (Lys; middle panels) of WT primary bone marrow-derived macrophages stimulated with LPS for the indicated times, followed by ATP (45 min) (A), or immortalized WT macrophages stimulated with LPS for 10 min in the presence or absence of cycloheximide (CHX), followed by ATP (45 min) (C). B, immunoblot of caspase-1 in the culture supernatants (upper panel) or cell lysates (middle panel) of WT macrophages unstimulated (Untreated; first lane) or stimulated with LPS (45 min; second lane), ATP (45 min; third lane), LPS for 10 min followed by ATP for 35 min (fourth lane), ATP for 10 min followed by LPS for 35 min (fifth lane), or simultaneously with LPS and ATP for 45 min (sixth lane). The lower panels in A–C show immunoblots of NLRP3 in the cell lysates of the same macrophages. Procasp-1, procaspase-1.
FIGURE 2.
Time course analysis of NLRP3 activation in WT and stable NLRP3-reconstituted _Nlrp3_-KO macrophages. A and B, immunoblots of caspase-1 in the culture supernatants (Sup; upper panels) of WT, N1-8, and NG5 macrophages stimulated with ATP alone for the indicated times (A) or stimulated with LPS for 10 min followed by ATP for the indicated times (B). The middle and lower panels show immunoblots of caspase-1 and NLRP3 in the cell lysates (Lys) of the same macrophages. Procasp-1, procaspase-1.
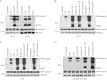
FIGURE 3.
Intact TLR4 signaling and mtROS are required for non-transcriptional priming of NLRP3. A, immunoblots of caspase-1 in the culture supernatants (Sup) of N1-8 (left panels) and NG5 (right panels) macrophages stimulated with LPS (10 min) plus ATP (45 min) or with ATP alone (45 min) in the presence or absence of NAC as indicated. B–D, immunoblots of caspase-1 in the culture supernatants of N1-8 (B), WT (C), and Myd88/_Trif_-dKO (D) macrophages stimulated with LPS, rotenone, or pyridaben for 10 min followed by ATP as indicated. The middle and lower panels in A–D show immunoblots of caspase-1 and NLRP3 in the cell lysates (Lys) of the same macrophages. Procasp-1, procaspase-1.
FIGURE 4.
Deubiquitination of NLRP3 is required for its priming and activation. A, B, and F, anti-ubiquitin (UB) antibody (upper panels) and anti-NLRP3 antibody (lower panels) immunoblots of anti-FLAG immunoprecipitates from _Nlrp3_-KO and N1-8 macrophages (A, first and second lanes, respectively) and N1-8 macrophages stimulated with LPS, ATP, or LPS plus ATP in the presence or absence of the deubiquitination inhibitors PR-619 and WP1130 (B) and the antioxidant NAC (F) as indicated. C, immunoblots of caspase-1 in the culture supernatants of N1-8 and NG5 macrophages stimulated with LPS plus ATP (N1-8) or ATP (NG5) in the presence or absence of PR-619 or WP1130 as indicated (upper panels). The lower panels show immunoblots of caspase-1 in the cell lysates of the same macrophages. D, anti-ubiquitin antibody (upper panel) and anti-NLRP3 antibody (middle panel) immunoblots of anti-FLAG immunoprecipitates from 293T-caspase-1-ASC (293T-CA) and 293T-caspase-1-ASC-NLRP3 (293T-CAN) cells stimulated with nigericin (Nig) for 60 min or left untreated (Un). The lower panel shows a caspase-1 immunoblot in the culture supernatants of the same cells. E, immunoblot of caspase-1 in cell lysates of 293T-caspase-1-ASC-NLRP3 cells treated with nigericin in the presence or absence of PR-619 as indicated. Procasp-1, procaspase-1.
Similar articles
- Molecular hydrogen inhibits lipopolysaccharide-triggered NLRP3 inflammasome activation in macrophages by targeting the mitochondrial reactive oxygen species.
Ren JD, Wu XB, Jiang R, Hao DP, Liu Y. Ren JD, et al. Biochim Biophys Acta. 2016 Jan;1863(1):50-5. doi: 10.1016/j.bbamcr.2015.10.012. Epub 2015 Oct 18. Biochim Biophys Acta. 2016. PMID: 26488087 - Both bone marrow-derived and non-bone marrow-derived cells contribute to AIM2 and NLRP3 inflammasome activation in a MyD88-dependent manner in dietary steatohepatitis.
Csak T, Pillai A, Ganz M, Lippai D, Petrasek J, Park JK, Kodys K, Dolganiuc A, Kurt-Jones EA, Szabo G. Csak T, et al. Liver Int. 2014 Oct;34(9):1402-13. doi: 10.1111/liv.12537. Epub 2014 Apr 17. Liver Int. 2014. PMID: 24650018 Free PMC article. - Mechanisms that lead to the regulation of NLRP3 inflammasome expression and activation in human dental pulp fibroblasts.
Zhang A, Wang P, Ma X, Yin X, Li J, Wang H, Jiang W, Jia Q, Ni L. Zhang A, et al. Mol Immunol. 2015 Aug;66(2):253-62. doi: 10.1016/j.molimm.2015.03.009. Epub 2015 Apr 2. Mol Immunol. 2015. PMID: 25863775 - The role of mitochondria in NLRP3 inflammasome activation.
Liu Q, Zhang D, Hu D, Zhou X, Zhou Y. Liu Q, et al. Mol Immunol. 2018 Nov;103:115-124. doi: 10.1016/j.molimm.2018.09.010. Epub 2018 Sep 21. Mol Immunol. 2018. PMID: 30248487 Review. - Regulation and Function of the Nucleotide Binding Domain Leucine-Rich Repeat-Containing Receptor, Pyrin Domain-Containing-3 Inflammasome in Lung Disease.
Lee S, Suh GY, Ryter SW, Choi AM. Lee S, et al. Am J Respir Cell Mol Biol. 2016 Feb;54(2):151-60. doi: 10.1165/rcmb.2015-0231TR. Am J Respir Cell Mol Biol. 2016. PMID: 26418144 Free PMC article. Review.
Cited by
- IRF3 function and immunological gaps in sepsis.
Basak B, Akashi-Takamura S. Basak B, et al. Front Immunol. 2024 Feb 5;15:1336813. doi: 10.3389/fimmu.2024.1336813. eCollection 2024. Front Immunol. 2024. PMID: 38375470 Free PMC article. Review. - Just say NO to NLRP3.
Rayamajhi M, Miao EA. Rayamajhi M, et al. Nat Immunol. 2013 Jan;14(1):12-4. doi: 10.1038/ni.2493. Nat Immunol. 2013. PMID: 23238751 No abstract available. - The Immunomodulatory Metabolite Itaconate Modifies NLRP3 and Inhibits Inflammasome Activation.
Hooftman A, Angiari S, Hester S, Corcoran SE, Runtsch MC, Ling C, Ruzek MC, Slivka PF, McGettrick AF, Banahan K, Hughes MM, Irvine AD, Fischer R, O'Neill LAJ. Hooftman A, et al. Cell Metab. 2020 Sep 1;32(3):468-478.e7. doi: 10.1016/j.cmet.2020.07.016. Epub 2020 Aug 12. Cell Metab. 2020. PMID: 32791101 Free PMC article. - Interactions between the NLRP3-Dependent IL-1β and the Type I Interferon Pathways in Human Plasmacytoid Dendritic Cells.
Bencze D, Fekete T, Pfliegler W, Szöőr Á, Csoma E, Szántó A, Tarr T, Bácsi A, Kemény L, Veréb Z, Pázmándi K. Bencze D, et al. Int J Mol Sci. 2022 Oct 12;23(20):12154. doi: 10.3390/ijms232012154. Int J Mol Sci. 2022. PMID: 36293012 Free PMC article. - Regulation of inflammasome activation.
Man SM, Kanneganti TD. Man SM, et al. Immunol Rev. 2015 May;265(1):6-21. doi: 10.1111/imr.12296. Immunol Rev. 2015. PMID: 25879280 Free PMC article. Review.
References
- Strowig T., Henao-Mejia J., Elinav E., Flavell R. (2012) Inflammasomes in health and disease. Nature 481, 278–286 - PubMed
- Aksentijevich I., Putnam C. D., Remmers E. F., Mueller J. L., Le J., Kolodner R. D., Moak Z., Chuang M., Austin F., Goldbach-Mansky R., Hoffman H. M., Kastner D. L. (2007) The clinical continuum of cryopyrinopathies: novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis Rheum. 56, 1273–1285 - PMC - PubMed
- Tschopp J., Schroder K. (2010) NLRP3 inflammasome activation: the convergence of multiple signaling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases