Highly stretchable and tough hydrogels - PubMed (original) (raw)
Highly stretchable and tough hydrogels
Jeong-Yun Sun et al. Nature. 2012.
Abstract
Hydrogels are used as scaffolds for tissue engineering, vehicles for drug delivery, actuators for optics and fluidics, and model extracellular matrices for biological studies. The scope of hydrogel applications, however, is often severely limited by their mechanical behaviour. Most hydrogels do not exhibit high stretchability; for example, an alginate hydrogel ruptures when stretched to about 1.2 times its original length. Some synthetic elastic hydrogels have achieved stretches in the range 10-20, but these values are markedly reduced in samples containing notches. Most hydrogels are brittle, with fracture energies of about 10 J m(-2) (ref. 8), as compared with ∼1,000 J m(-2) for cartilage and ∼10,000 J m(-2) for natural rubbers. Intense efforts are devoted to synthesizing hydrogels with improved mechanical properties; certain synthetic gels have reached fracture energies of 100-1,000 J m(-2) (refs 11, 14, 17). Here we report the synthesis of hydrogels from polymers forming ionically and covalently crosslinked networks. Although such gels contain ∼90% water, they can be stretched beyond 20 times their initial length, and have fracture energies of ∼9,000 J m(-2). Even for samples containing notches, a stretch of 17 is demonstrated. We attribute the gels' toughness to the synergy of two mechanisms: crack bridging by the network of covalent crosslinks, and hysteresis by unzipping the network of ionic crosslinks. Furthermore, the network of covalent crosslinks preserves the memory of the initial state, so that much of the large deformation is removed on unloading. The unzipped ionic crosslinks cause internal damage, which heals by re-zipping. These gels may serve as model systems to explore mechanisms of deformation and energy dissipation, and expand the scope of hydrogel applications.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
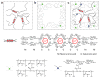
Figure 1. Schematics of three types of hydrogels
a, In an alginate gel, the G blocks on different polymer chains form ionic crosslinks through Ca2+. b, In a polyacrylamide gel, the polymer chains form covalent crosslinks through MBAA. c, In an alginate-polyacrylamide hybrid gel, the two types of polymer networks are intertwined.
Figure 2. The hybridgel is highly stretchable and notch-insensitive
a, A strip of the undeformed gel was glued to two rigid clamps. b, The gel was stretched 21 times its initial length. The stretch λ is defined by the distance between the two clamps when the gel is deformed divided by the distance when the gel is undeformed. c, A notch was cut into the gel by using a razor blade; a small stretch of 1.15 was used to make the notch clearly visible. d, The gel containing the notch was stretched 17 times its initial length. The alginate-to-acrylamide ratio was 1:8. The covalent crosslinker, MBAA, was fixed at 0.0006 the weight of acrylamide. The ionic crosslinker, CaSO4, was fixed at 0.1328 the weight of alginate.
Figure 3. Mechanical tests under various conditions
a, Stress-stretch curves of the three types of gels, each stretched to rupture. The nominal stress s is defined by the force applied on the deformed gel divided by the cross-sectional area of the undeformed gel. b, The gels were each loaded to a stretch of 1.2, just below the value that would rupture the alginate gel, and were then unloaded. c, Samples of the hybrid gel were subject to a cycle of loading and unloading of varying maximum stretch. d, After the first loading and unloading, one sample was reloaded immediately, and the other sample was reloaded after 1 day. e, Recovery of samples stored at 80°C for different durations of time. f, The work of the second loading W_2_nd normalized by that of the first loading W_1_st was measured for samples stored for different periods of time at different temperatures. The alginate-to-acrylamide ratio was 1:8 for a and b, and was 1:6 for c–f. The covalent crosslinker, MBAA, was fixed at 0.0006 the weight of acrylamide for polyacrylamide gel and hybrid gel. The ionic crosslinker, CaSO4, was fixed at 0.1328 the weight of alginate for alginate gel and hybrid gel.
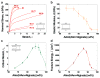
Figure 4. The composition greatly affects the behavior of the hybrid gel
a, Stress-strain curves of gels of various weight ratios of acrylamide and alginate. Each test was conducted by pulling an unnotched sample to rupture. b, Elastic moduli were calculated from stress-strain curves. c, Notched gels of various acrylamide-to-alginate ratios were pulled to rupture to measure the critical stretches. d, Fracture energy was plotted as a function of the acrylamide-to-alginate ratio. The covalent crosslinker, MBAA, was fixed at 0.0006 the weight of acrylamide. The ionic crosslinker, CaSO4, was fixed at 0.1328 the weight of alginate. (Error bars, S.D.; n=4)
Comment in
- Materials science: A hard concept in soft matter.
Shull KR. Shull KR. Nature. 2012 Sep 6;489(7414):36-7. doi: 10.1038/489036a. Nature. 2012. PMID: 22955605 No abstract available.
Similar articles
- Softening and Shape Morphing of Stiff Tough Hydrogels by Localized Unlocking of the Trivalent Ionically Cross-Linked Centers.
Wang J, Li T, Chen F, Zhou D, Li B, Zhou X, Gan T, Handschuh-Wang S, Zhou X. Wang J, et al. Macromol Rapid Commun. 2018 Jun;39(12):e1800143. doi: 10.1002/marc.201800143. Epub 2018 May 11. Macromol Rapid Commun. 2018. PMID: 29749078 - Strengthening alginate/polyacrylamide hydrogels using various multivalent cations.
Yang CH, Wang MX, Haider H, Yang JH, Sun JY, Chen YM, Zhou J, Suo Z. Yang CH, et al. ACS Appl Mater Interfaces. 2013 Nov 13;5(21):10418-22. doi: 10.1021/am403966x. Epub 2013 Oct 18. ACS Appl Mater Interfaces. 2013. PMID: 24128011 - Elastic, superporous hydrogel hybrids of polyacrylamide and sodium alginate.
Omidian H, Rocca JG, Park K. Omidian H, et al. Macromol Biosci. 2006 Sep 15;6(9):703-10. doi: 10.1002/mabi.200600062. Macromol Biosci. 2006. PMID: 16967483 - Interpenetrating Polymer Networks polysaccharide hydrogels for drug delivery and tissue engineering.
Matricardi P, Di Meo C, Coviello T, Hennink WE, Alhaique F. Matricardi P, et al. Adv Drug Deliv Rev. 2013 Aug;65(9):1172-87. doi: 10.1016/j.addr.2013.04.002. Epub 2013 Apr 17. Adv Drug Deliv Rev. 2013. PMID: 23603210 Review. - Tough Hydrogels with Different Toughening Mechanisms and Applications.
Xu Z, Chen Y, Cao Y, Xue B. Xu Z, et al. Int J Mol Sci. 2024 Feb 26;25(5):2675. doi: 10.3390/ijms25052675. Int J Mol Sci. 2024. PMID: 38473922 Free PMC article. Review.
Cited by
- In situ continuous hydrogen-bonded engineering for intrinsically stretchable and healable high-mobility polymer semiconductors.
Yue H, Wang Y, Luo S, Guo J, Jin J, Li G, Meng Z, Zhang L, Zhou D, Zhen Y, Hu W. Yue H, et al. Sci Adv. 2024 Oct 4;10(40):eadq0171. doi: 10.1126/sciadv.adq0171. Epub 2024 Oct 2. Sci Adv. 2024. PMID: 39356754 Free PMC article. - Research progress of cartilage lubrication and biomimetic cartilage lubrication materials.
An H, Liu Y, Yi J, Xie H, Li C, Wang X, Chai W. An H, et al. Front Bioeng Biotechnol. 2022 Oct 4;10:1012653. doi: 10.3389/fbioe.2022.1012653. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36267457 Free PMC article. Review. - Exceptionally Fast Temperature-Responsive, Mechanically Strong and Extensible Monolithic Non-Porous Hydrogels: Poly(_N_-isopropylacrylamide) Intercalated with Hydroxypropyl Methylcellulose.
Strachota B, Strachota A, Vratović L, Pavlova E, Šlouf M, Kamel S, Cimrová V. Strachota B, et al. Gels. 2023 Nov 24;9(12):926. doi: 10.3390/gels9120926. Gels. 2023. PMID: 38131912 Free PMC article. - Instant tough adhesion of polymer networks.
Freedman BR, Cintron Cruz JA, Kwon P, Lee M, Jeffers HM, Kent D, Wu KC, Weaver JC, Mooney DJ. Freedman BR, et al. Proc Natl Acad Sci U S A. 2024 Feb 27;121(9):e2304643121. doi: 10.1073/pnas.2304643121. Epub 2024 Feb 20. Proc Natl Acad Sci U S A. 2024. PMID: 38377210 Free PMC article. - Phase-change composite filled natural nanotubes in hydrogel promote wound healing under photothermally triggered drug release.
Ye JJ, Li LF, Hao RN, Gong M, Wang T, Song J, Meng QH, Zhao NN, Xu FJ, Lvov Y, Zhang LQ, Xue JJ. Ye JJ, et al. Bioact Mater. 2022 Sep 12;21:284-298. doi: 10.1016/j.bioactmat.2022.08.026. eCollection 2023 Mar. Bioact Mater. 2022. PMID: 36157247 Free PMC article.
References
- Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. - PubMed
- Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Ad Drug Delivery Rev. 2001;53:321–339. - PubMed
- Dong L, Agarwal AK, Beebe DJ, Jiang HR. Adaptive liquid microlenses activated by stimuli-responsive hydrogels. Nature. 2006;442:551–554. - PubMed
- Calvert P. Hydrogels for soft machines. Adv Mater. 2009;21:743–756.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources