Microbial shifts in the swine distal gut in response to the treatment with antimicrobial growth promoter, tylosin - PubMed (original) (raw)
Microbial shifts in the swine distal gut in response to the treatment with antimicrobial growth promoter, tylosin
Hyeun Bum Kim et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Antimicrobials have been used extensively as growth promoters (AGPs) in agricultural animal production. However, the specific mechanism of action for AGPs has not yet been determined. The work presented here was to determine and characterize the microbiome of pigs receiving one AGP, tylosin, compared with untreated pigs. We hypothesized that AGPs exerted their growth promoting effect by altering gut microbial population composition. We determined the fecal microbiome of pigs receiving tylosin compared with untreated pigs using pyrosequencing of 16S rRNA gene libraries. The data showed microbial population shifts representing both microbial succession and changes in response to the use of tylosin. Quantitative and qualitative analyses of sequences showed that tylosin caused microbial population shifts in both abundant and less abundant species. Our results established a baseline upon which mechanisms of AGPs in regulation of health and growth of animals can be investigated. Furthermore, the data will aid in the identification of alternative strategies to improve animal health and consequently production.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
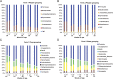
RDP classification of the sequences at phylum and class levels. RDP classifier was used with a bootstrap cutoff of 50. Pooled sequence reads from all 10 pigs in each group were used. (A) RDP classification of the sequence reads from farm 1 at phylum level. (B) RDP classification of the sequence reads from farm 2 at phylum level. (C) RDP classification of the sequence reads from farm 1 at class level. (D) RDP classification of the sequence reads from farm 2 at class level.
Fig. 2.
Differentially abundant genera between tylosin and no-tylosin groups. Pigs in the tylosin groups received tylosin in their feed during the whole experimental period (10–22 wk of age), whereas pigs in the no-tylosin groups did not receive tylosin. The heat map was created using genus based results after normalization. Yellow indicates abundant genera and black indicates less abundant genera. Each column represents groups and each row indicates genus. F1 and F2 indicate farm 1 and farm 2, respectively, and T and NT represent each tylosin group and no-tylosin group. (A) Differentially abundant genera were indicated by arrows and color-coded. Genus names indicate by the color their relationship to the phylum level. (B) Twelve differentially abundant genera and unclassified genera.
Fig. 3.
Distribution of OTUs in the two treatment groups in farm 1. The heat map for farm 1 was created using OTUs with an OTU definition at a similarity cutoff of 95%. Each column represents groups and each row indicates OTUs. OTUs were sorted with the most abundant OTUs displayed at the top and the least abundant OTUs at the bottom. T and NT indicate tylosin and no-tylosin group, respectively, and numbers indicates weeks of age. Abundant OTUs were red color-coded and white blanks indicate missing OTUs.
Fig. 4.
Distribution of OTUs in the two treatment groups in the farm 2. The heat map for the farm 2 was created using OTUs with an OTU definition at a similarity cutoff of 93%. Each column represents groups and each row indicates OTUs. OTUs were sorted with the most abundant OTUs displayed at the top and the least abundant OTUs at the bottom. T and NT indicate tylosin and no-tylosin group, respectively, and numbers indicates weeks of age. Abundant OTUs were red color-coded and white blanks indicate missing OTUs.
Similar articles
- Response of intestinal microbiota to antibiotic growth promoters in chickens.
Lin J, Hunkapiller AA, Layton AC, Chang YJ, Robbins KR. Lin J, et al. Foodborne Pathog Dis. 2013 Apr;10(4):331-7. doi: 10.1089/fpd.2012.1348. Epub 2013 Mar 5. Foodborne Pathog Dis. 2013. PMID: 23461609 - Effects of the Antibiotics Growth Promoter Tylosin on Swine Gut Microbiota.
Kim J, Guevarra RB, Nguyen SG, Lee JH, Jeong DK, Unno T. Kim J, et al. J Microbiol Biotechnol. 2016 May 28;26(5):876-82. doi: 10.4014/jmb.1512.12004. J Microbiol Biotechnol. 2016. PMID: 26869601 - Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment.
Danzeisen JL, Kim HB, Isaacson RE, Tu ZJ, Johnson TJ. Danzeisen JL, et al. PLoS One. 2011;6(11):e27949. doi: 10.1371/journal.pone.0027949. Epub 2011 Nov 16. PLoS One. 2011. PMID: 22114729 Free PMC article. - Enterotypes in the landscape of gut microbial community composition.
Costea PI, Hildebrand F, Arumugam M, Bäckhed F, Blaser MJ, Bushman FD, de Vos WM, Ehrlich SD, Fraser CM, Hattori M, Huttenhower C, Jeffery IB, Knights D, Lewis JD, Ley RE, Ochman H, O'Toole PW, Quince C, Relman DA, Shanahan F, Sunagawa S, Wang J, Weinstock GM, Wu GD, Zeller G, Zhao L, Raes J, Knight R, Bork P. Costea PI, et al. Nat Microbiol. 2018 Jan;3(1):8-16. doi: 10.1038/s41564-017-0072-8. Epub 2017 Dec 18. Nat Microbiol. 2018. PMID: 29255284 Free PMC article. Review. - Antimicrobial use in swine production and its effect on the swine gut microbiota and antimicrobial resistance.
Holman DB, Chénier MR. Holman DB, et al. Can J Microbiol. 2015 Nov;61(11):785-98. doi: 10.1139/cjm-2015-0239. Epub 2015 Aug 20. Can J Microbiol. 2015. PMID: 26414105 Review.
Cited by
- Antimicrobial promotion of pig growth is associated with tissue-specific remodeling of bile acid signature and signaling.
Ipharraguerre IR, Pastor JJ, Gavaldà-Navarro A, Villarroya F, Mereu A. Ipharraguerre IR, et al. Sci Rep. 2018 Sep 12;8(1):13671. doi: 10.1038/s41598-018-32107-9. Sci Rep. 2018. PMID: 30209339 Free PMC article. - Altered fecal microbiota composition associated with food allergy in infants.
Ling Z, Li Z, Liu X, Cheng Y, Luo Y, Tong X, Yuan L, Wang Y, Sun J, Li L, Xiang C. Ling Z, et al. Appl Environ Microbiol. 2014 Apr;80(8):2546-54. doi: 10.1128/AEM.00003-14. Epub 2014 Feb 14. Appl Environ Microbiol. 2014. PMID: 24532064 Free PMC article. - Gut microbiome perturbation and its correlation with tylosin pharmacokinetics in healthy and infected pigs.
Lee EB, Lee GY, Hossain MA, Awji EG, Park SC. Lee EB, et al. Sci Rep. 2024 Aug 12;14(1):18670. doi: 10.1038/s41598-024-69566-2. Sci Rep. 2024. PMID: 39134586 Free PMC article. - Maintenance of gut microbiome stability for optimum intestinal health in pigs - a review.
Upadhaya SD, Kim IH. Upadhaya SD, et al. J Anim Sci Biotechnol. 2022 Dec 7;13(1):140. doi: 10.1186/s40104-022-00790-4. J Anim Sci Biotechnol. 2022. PMID: 36474259 Free PMC article. Review. - Lawsonia intracellularis: Revisiting the Disease Ecology and Control of This Fastidious Pathogen in Pigs.
Karuppannan AK, Opriessnig T. Karuppannan AK, et al. Front Vet Sci. 2018 Aug 9;5:181. doi: 10.3389/fvets.2018.00181. eCollection 2018. Front Vet Sci. 2018. PMID: 30140680 Free PMC article. Review.
References
- Jones FT, Ricke SC. Observations on the history of the development of antimicrobials and their use in poultry feeds. Poult Sci. 2003;82:613–617. - PubMed
- Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: History and mode of action. Poult Sci. 2005;84:634–643. - PubMed
- Niewold TA. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult Sci. 2007;86:605–609. - PubMed
- McEwen SA, Fedorka-Cray PJ. Antimicrobial use and resistance in animals. Clin Infect Dis. 2002;34(Suppl 3):S93–S106. - PubMed
- Dewey CE, Cox BD, Straw BE, Bush EJ. Use of antimicrobials in swine feeds in the United States. Swine Health and Production. 1999;7:19–25.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources