Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer's disease pathology - PubMed (original) (raw)
Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer's disease pathology
Jee Hoon Roh et al. Sci Transl Med. 2012.
Abstract
Aggregation of β-amyloid (Aβ) in the brain begins to occur years before the clinical onset of Alzheimer's disease (AD). Before Aβ aggregation, concentrations of extracellular soluble Aβ in the interstitial fluid (ISF) space of the brain, which are regulated by neuronal activity and the sleep-wake cycle, correlate with the amount of Aβ deposition in the brain seen later. The amount and quality of sleep decline with normal aging and to a greater extent in AD patients. How sleep quality as well as the diurnal fluctuation in Aβ change with age and Aβ aggregation is not well understood. We report a normal sleep-wake cycle and diurnal fluctuation in ISF Aβ in the brain of the APPswe/PS1δE9 mouse model of AD before Aβ plaque formation. After plaque formation, the sleep-wake cycle markedly deteriorated and diurnal fluctuation of ISF Aβ dissipated. As in mice, diurnal fluctuation of cerebrospinal fluid Aβ in young adult humans with presenilin mutations was also markedly attenuated after Aβ plaque formation. Virtual elimination of Aβ deposits in the mouse brain by active immunization with Aβ(42) normalized the sleep-wake cycle and the diurnal fluctuation of ISF Aβ. These data suggest that Aβ aggregation disrupts the sleep-wake cycle and diurnal fluctuation of Aβ. Sleep-wake behavior and diurnal fluctuation of Aβ in the central nervous system may be functional and biochemical indicators, respectively, of Aβ-associated pathology.
Figures
Figure 1
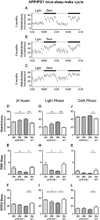
Chronological changes in sleep-wake patterns and diurnal fluctuations of interstitial fluid (ISF) amyloid beta (Aβ) in APPswe/PS1δE9 mice. (A–C, G–I) Diurnal changes of ISF Aβ1-x in APPswe/PS1δE9 mice at 3, 6, 9 months across 2 days shown as % average of 2 days of absolute values of ISF Aβ1-x in the hippocampus (A–C) and striatum (G–I). (D–F, J–L) Comparison of % average of 2 days of ISF Aβ1-x between dark and light periods in the hippocampus (D–F) and striatum (J–L) of each age group (n = 6–8 per group; two tailed t-test). (M, N) Absolute levels of ISF Aβ1-x in the hippocampus (M) and striatum (N) of 3, 6, and 9 month old APPswe/PS1δE9 mice (n = 6–8 per group; one-way ANOVA, followed by Tukey’s post hoc test). *P < 0.05; **P <0.01; ***P< 0.001. Values represent mean ± s.e.m.
Figure 2
Sleep-wake patterns in 3, 6, and 9 month old APPswe/PS1δE9 mice. (A–C) Sleep-wake patterns in 3, 6, and 9 month old APPswe/PS1δE9 mice across 2 days (2 light-dark periods) assessed as minutes awake per hour. (D–L) Chronological changes of minutes per hour spent in wakefulness, rapid eye movement (REM) sleep, and non-REM (NREM) sleep in 3, 6, and 9 month old APPswe/PS1δE9 mice and 9 month old wild-type littermates (B6C3). Analysis of a whole 24 hour period (D–F), analysis during light period (G–I), and analysis during dark period (J–L) (n = 6–8 per group; one-way ANOVA, Tukey’s post hoc test for multiple comparisons). *P < 0.05; **P<0.01; ***p < 0.001. Values represent mean ± s.e.m.
Figure 3
Attenuated diurnal fluctuation of CSF Aβ in mutation carriers in autosomal dominant Alzheimer’s disease (AD) families. (A–F) Diurnal fluctuation of CSF ISF Aβ40 and Aβ42 across 36 hours in no mutation carriers (mutation−; N=4) (A, D), mutation carriers who are PiB−(mutation+PiB−; N=4) (B, E) and mutation carriers who are PiB+ (mutation+PiB+; N=4) (C, F) as shown by cosinor curves. Cosinor analysis was used to assess diurnal patterns of CSF Aβ dynamics in each group and diurnal patterns were considered significant when amplitudes were different from zero (P < 0.05).
Figure 4
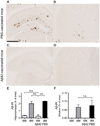
Aβ plaque deposition in the hippocampus and striatum in 9 month old phosphate buffered saline (PBS)-treated and Aβ42-immunized APPswe/PS1δE9 mice. (A–D) Representative brain sections of the hippocampus (A, C) and striatum (B, D) of mice from each group stained with HJ 3.4 antibody to visualize Aβ immunoreactive plaques (Aβ-IR). (E, F) Amount of Aβ deposition in the PBS-treated mice and Aβ42-vaccinated mice are shown with amount of Aβ deposition in six and nine month old APPswe/PS1δE9 mice in the hippocampus (E) and striatum (F) (n=5–6 in each group; two tailed t-test). ***P< 0.001. n.s. stands for not statistically significant. Values represent mean ± s.e.m. Scale bar in (A) represents 500µm.
Figure 5
Sleep-wake patterns and diurnal fluctuation of interstitial fluid (ISF) Aβ in 9 month old phosphate buffered saline (PBS)-treated and Aβ42-immunized APPswe/PS1δE9 mice. (A, G) Sleep-wake patterns in 9 month old PBS-treated (A) and Aβ42-immunized (G) APPswe/PS1δE9 mice across 2 days (2 light-dark periods) shown as minutes awake per hour. (D, J) Comparison of minutes awake per hour between the dark and light periods in each group (n = 5–6 per group; two tailed t-test). (B, H) Diurnal fluctuation of ISF Aβ1-x in the hippocampus of 9 month old PBS-treated (B) and Aβ42-immunized (H) APPswe/PS1δE9 mice across 2 days presented as % average of absolute values of ISF Aβ1-x**(E, K)** Comparison of % average of absolute values of ISF Aβ1-x in the hippocampus between the dark and light periods (n = 5–6 per group; two tailed t-test). (C, I) Diurnal fluctuation of ISF Aβ1-x in the striatum of 9 month old PBS-vaccinated (C) and Aβ42-vaccinated (I) APPswe/PS1δE9 mice across 2 days. (F, L) Comparison of % average of absolute values of ISF Aβ1-x in the striatum between the dark and light periods (n = 5–6 per group; two tailed t-test). *P < 0.05; ***p < 0.001. Values represent mean ± s.e.m.
Figure 6
Chronological changes in the amplitude of diurnal fluctuation of interstitial fluid (ISF) lactate in 3, 6, and 9 month old APPswe/PS1δE9 mice. (A–F) Diurnal fluctuation of ISF lactate in the hippocampus (A–C) and in the striatum (D–F). (G, H) Chronological changes in the amplitude of diurnal fluctuation in the hippocampus (G) and in the striatum (H) as measured by amplitude of cosinor analysis (n = 6 per group; one-way ANOVA after cosinor analysis for measurement of amplitude, Tukey’s post hoc test for multiple comparisons). *P < 0.05. Values represent mean ± s.e.m.
Figure 7
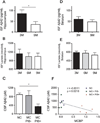
Chronological changes of absolute concentrations of interstitial fluid (ISF) Aβ42 and lactate in the hippocampus and striatum of mice and association between CSF Aβ42 and amyloid plaque deposition in humans. (A, D) Absolute levels of ISF Aβx-42 in the hippocampus (A) and in the striatum (D) of 3 and 9 month old APPswe/PS1δE9 mice (n = 5–6 per group; Mann-Whitney Test). (B, E) Absolute levels of ISF lactate in the hippocampus (B) and in the striatum (E) of 3, 6, and 9 month old APPswe/PS1δE9 mice (n = 6 per group; one-way ANOVA, Tukey’s_post hoc_ test). (C) Comparison of absolute values of CSF Aβ42 in non-mutation carriers (NC), mutation carriers without amyloid plaque deposition (MC+PiB−), mutation carriers with amyloid plaque deposition (MC+PiB+) (n = 4 per group; Kruskal-Wallis test). (F) Correlation between absolute levels of CSF Aβ42 and amount of amyloid plaque deposition measured by mean cortical PiB binding potential (MCBP) (n = 12 paired measurement; Pearson’s correlation test). *P < 0.05; **P < 0.01. Values represent mean ± s.e.m.
Comment in
- The nexus of Aβ, aging, and sleep.
Gerstner JR, Perron IJ, Pack AI. Gerstner JR, et al. Sci Transl Med. 2012 Sep 5;4(150):150fs34. doi: 10.1126/scitranslmed.3004815. Sci Transl Med. 2012. PMID: 22956197 Free PMC article.
Similar articles
- Effects of growth hormone-releasing hormone on sleep and brain interstitial fluid amyloid-β in an APP transgenic mouse model.
Liao F, Zhang TJ, Mahan TE, Jiang H, Holtzman DM. Liao F, et al. Brain Behav Immun. 2015 Jul;47:163-71. doi: 10.1016/j.bbi.2014.09.005. Epub 2014 Sep 16. Brain Behav Immun. 2015. PMID: 25218899 Free PMC article. - Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle.
Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Kang JE, et al. Science. 2009 Nov 13;326(5955):1005-7. doi: 10.1126/science.1180962. Epub 2009 Sep 24. Science. 2009. PMID: 19779148 Free PMC article. - The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans.
Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, Finn MB, Manis M, Geerling JC, Fuller PM, Lucey BP, Holtzman DM. Holth JK, et al. Science. 2019 Feb 22;363(6429):880-884. doi: 10.1126/science.aav2546. Epub 2019 Jan 24. Science. 2019. PMID: 30679382 Free PMC article. - Amyloid-β diurnal pattern: possible role of sleep in Alzheimer's disease pathogenesis.
Lucey BP, Bateman RJ. Lucey BP, et al. Neurobiol Aging. 2014 Sep;35 Suppl 2:S29-34. doi: 10.1016/j.neurobiolaging.2014.03.035. Epub 2014 May 15. Neurobiol Aging. 2014. PMID: 24910393 Review. - The Emerging Relationship Between Interstitial Fluid-Cerebrospinal Fluid Exchange, Amyloid-β, and Sleep.
Boespflug EL, Iliff JJ. Boespflug EL, et al. Biol Psychiatry. 2018 Feb 15;83(4):328-336. doi: 10.1016/j.biopsych.2017.11.031. Epub 2017 Dec 7. Biol Psychiatry. 2018. PMID: 29279202 Free PMC article. Review.
Cited by
- The relationship between Alzheimer's-related brain atrophy patterns and sleep macro-architecture.
Weihs A, Frenzel S, Garvert L, Kühn L, Wittfeld K, Ewert R, Fietze I, Penzel T, Stubbe B, Szentkirályi A, Wulms N, Völzke H, Grabe HJ. Weihs A, et al. Alzheimers Dement (Amst). 2022 Nov 11;14(1):e12371. doi: 10.1002/dad2.12371. eCollection 2022. Alzheimers Dement (Amst). 2022. PMID: 36381559 Free PMC article. - β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation.
Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ, Walker MP. Mander BA, et al. Nat Neurosci. 2015 Jul;18(7):1051-7. doi: 10.1038/nn.4035. Epub 2015 Jun 1. Nat Neurosci. 2015. PMID: 26030850 Free PMC article. - Increased in vivo amyloid-β42 production, exchange, and loss in presenilin mutation carriers.
Potter R, Patterson BW, Elbert DL, Ovod V, Kasten T, Sigurdson W, Mawuenyega K, Blazey T, Goate A, Chott R, Yarasheski KE, Holtzman DM, Morris JC, Benzinger TL, Bateman RJ. Potter R, et al. Sci Transl Med. 2013 Jun 12;5(189):189ra77. doi: 10.1126/scitranslmed.3005615. Sci Transl Med. 2013. PMID: 23761040 Free PMC article. - Circadian clock disruption in neurodegenerative diseases: cause and effect?
Musiek ES. Musiek ES. Front Pharmacol. 2015 Feb 27;6:29. doi: 10.3389/fphar.2015.00029. eCollection 2015. Front Pharmacol. 2015. PMID: 25774133 Free PMC article. - Genetic Risk of Alzheimer's Disease and Sleep Duration in Non-Demented Elders.
Leng Y, Ackley SF, Glymour MM, Yaffe K, Brenowitz WD. Leng Y, et al. Ann Neurol. 2021 Jan;89(1):177-181. doi: 10.1002/ana.25910. Epub 2020 Oct 5. Ann Neurol. 2021. PMID: 32951248 Free PMC article.
References
- Selkoe DJ. Alzheimer's disease:genes proteins and therapy. Physiological reviews. 2001 Apr;81:741. - PubMed
- Golde TE, Eckman CB, Younkin SG. Biochemical detection of Abeta isoforms: implications for pathogenesis diagnosis and treatment of Alzheimer's disease. Biochimica et biophysica acta. 2000 Jul 26;1502:172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS057105/NS/NINDS NIH HHS/United States
- R01NS065667/NS/NINDS NIH HHS/United States
- K23AG03094604/AG/NIA NIH HHS/United States
- P01NS074969/NS/NINDS NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- AG13956/AG/NIA NIH HHS/United States
- K23 AG030946/AG/NIA NIH HHS/United States
- R01 AG013956/AG/NIA NIH HHS/United States
- P01AG026276-S1/AG/NIA NIH HHS/United States
- R01 NS065667/NS/NINDS NIH HHS/United States
- P01 NS074969/NS/NINDS NIH HHS/United States
- R37 AG013956/AG/NIA NIH HHS/United States
- P30NS057105/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials