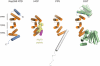Crystal structure of the N-terminal domain of Nup358/RanBP2 - PubMed (original) (raw)
Crystal structure of the N-terminal domain of Nup358/RanBP2
Susanne A Kassube et al. J Mol Biol. 2012.
Abstract
Key steps in mRNA export are the nuclear assembly of messenger ribonucleoprotein particles (mRNPs), the translocation of mRNPs through the nuclear pore complex (NPC), and the mRNP remodeling events at the cytoplasmic side of the NPC. Nup358/RanBP2 is a constituent of the cytoplasmic filaments of the NPC specific to higher eukaryotes and provides a multitude of binding sites for the nucleocytoplasmic transport machinery. Here, we present the crystal structure of the Nup358 N-terminal domain (NTD) at 0.95Å resolution. The structure reveals an α-helical domain that harbors three central tetratricopeptide repeats (TPRs), flanked on each side by an additional solvating amphipathic α helix. Overall, the NTD adopts an unusual extended conformation that lacks the characteristic peptide-binding groove observed in canonical TPR domains. Strikingly, the vast majority of the NTD surface exhibits an evolutionarily conserved, positive electrostatic potential, and we demonstrate that the NTD possesses the capability to bind single-stranded RNA in solution. Together, these data suggest that the NTD contributes to mRNP remodeling events at the cytoplasmic face of the NPC.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Fig. 1
Biochemical and biophysical analysis of Nup358NTD. (a) Domain organization of human Nup358. Domain boundaries are indicated by residue numbers. The bar above the domain structure denotes the crystallized fragment. I, II, III, and IV, Ran binding domains; NTD, N-terminal domain; CycH, cyclophilin homology domain; E3, E3 ligase domain. (b) Multiangle light-scattering (MALS) analysis of the P. troglodytes Nup358NTD. The differential refractive index is plotted against the elution volumes from a Superdex 200 10/300 GL gel-filtration column and is overlaid with the determined molecular mass.
Fig. 2
Multi-species sequence alignment of Nup358NTD. The overall sequence conservation is shaded from white (less than 60 % similarity) to red (100 % identity) and has been calculated based on the Blosum62 algorithm. The secondary structure as observed in Nup358NTD structure is shown above the alignment, with blue bars representing α helices. The numbering below the sequence is relative to human Nup358. The positions of the introduced methinonine mutants and Y52 are indicated by green and purple dots, respectively.
Fig. 3
Structure of the NTD of Nup358. (a) Ribbon representation of Nup358NTD shown in rainbow colors from the N- to the C-terminus (αA to αH). Views rotated by 90°, 180°, and 270° are shown on the right. (b) Schematic drawing showing the structural organization of Nup358NTD. The α helices of the three centrally positioned TPR repeats form two layers, one on the concave (αB, αD, and αF) and one on the convex side (αC, αE, and αG) of the domain. The TPRs are capped by solvating α helices on both termini. (c) Representative final 2|fo|-|fc| electron density maps, rendered at 5.0 σ.
Fig. 4
The non-canonical conformation of Nup358NTD is induced by bulky hydrophobic side chains. (a) Cartoon representation of Nup358NTD with selected side chains shown in ball and stick. A 90°-rotated view is shown on the right. (b) Helical wheel representation of the Nup358NTD with selected hydrophobic side chains drawn. The star denotes the position of the conserved Tyr52 in helix αC that induces the kink in helix αB.
Fig. 5
The TPR motif of Nup358NTD. (a) Superposition of the three TPRs of Nup358NTD. The positions of the Cα atoms are shown as blue spheres. A 90°-rotated view is shown on the right. The three TPRs align well with the exception of the kinked α helix αB of TPR1, and the αF-αG loop of TPR3, which is one residue longer than in a canonical TPR. (b) Structure-based sequence alignment of the three TPRs and the two solvating α helices. The sequence of the consensus TPR is shown at the bottom of the alignment and is based on the analysis by Main and coworkers. The position of the α helices and the connecting loops relative to the consensus sequence is indicated at the top. Conserved small and large hydrophobic residues are highlighted with grey bars; large hydrophobic side chains are highlighted in orange.
Fig. 6
Surface properties of Nup358NTD. (a) Surface representation illustrating the surface conservation. The surface is colored according to a multi-species sequence alignment (Fig. 2). The conservation at each position is mapped onto the surface and is shaded in a color gradient from white (60 % similarity) to red (100 % identity). (b) Surface rendition colored according to the electrostatic potential from -5 kBT/e (red) to +5 kBT/e (blue). Black lines indicate the borders of the two evolutionarily conserved hydrophobic patches.
Fig. 7
Comparison of Nup358NTD to other TPR proteins. The three central TPR repeats of (A) Nup358NTD, (B) the TPR1 domain of Hop in complex with an Hsp70 peptide (PDB code 1ELW), (C) the TPR domain of protein phosphatase 5 (PDB code 1A17), and (D) the TPR domain of O-linked GlcNAc transferase (PDB code 1W3B) are illustrated. Equivalent TPRs are colored in orange. The Hsp70 peptide bound to the TPR1 domain of Hop is shown in yellow. Nup358NTD adopts a substantially shallower conformation as compared to the other TPR proteins.
Fig. 8
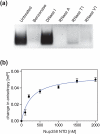
Nup358NTD interacts with single-stranded RNA. (a) Nup358NTD co-purifies with E. coli RNA. Purified Nup358NTD•RNA was subjected to various DNA and RNA nuclease digests. (b) Association of Nup358NTD with fluorescently-labeled, single-stranded, degenerate, decameric RNA oligo-nucleotide. Fluorescence polarization reveals an apparent dissociation constant (Kd) of 264±45 μM between Nup358NTD and a Cy3-labeled degenerate, decameric, single-stranded RNA oligonucleotide. The mean of three experiments each measured three times are represented, error bars represent the SEM (standard error of the mean).
Fig 9
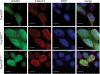
In vivo localization of full-length Nup358 and Nup358 fragments. HEK293T cells were transfected with Nup358 and Nup358 fragments containing a C-terminal HA-tag and analyzed by fluorescence microscopy. The cellular localization of the HA-tagged Nup358 proteins was detected with an α-HA antibody (green). The monoclonal α-mAb414 antibody (red) and DAPI (blue) were used as a reference for nuclear envelope and nucleus staining, respectively. The merged image is shown on the right.
Similar articles
- Structural and functional analysis of the C-terminal domain of Nup358/RanBP2.
Lin DH, Zimmermann S, Stuwe T, Stuwe E, Hoelz A. Lin DH, et al. J Mol Biol. 2013 Apr 26;425(8):1318-29. doi: 10.1016/j.jmb.2013.01.021. Epub 2013 Jan 23. J Mol Biol. 2013. PMID: 23353830 Free PMC article. - Architecture of the cytoplasmic face of the nuclear pore.
Bley CJ, Nie S, Mobbs GW, Petrovic S, Gres AT, Liu X, Mukherjee S, Harvey S, Huber FM, Lin DH, Brown B, Tang AW, Rundlet EJ, Correia AR, Chen S, Regmi SG, Stevens TA, Jette CA, Dasso M, Patke A, Palazzo AF, Kossiakoff AA, Hoelz A. Bley CJ, et al. Science. 2022 Jun 10;376(6598):eabm9129. doi: 10.1126/science.abm9129. Epub 2022 Jun 10. Science. 2022. PMID: 35679405 Free PMC article. - Structural and functional analysis of the interaction between the nucleoporin Nup98 and the mRNA export factor Rae1.
Ren Y, Seo HS, Blobel G, Hoelz A. Ren Y, et al. Proc Natl Acad Sci U S A. 2010 Jun 8;107(23):10406-11. doi: 10.1073/pnas.1005389107. Epub 2010 May 24. Proc Natl Acad Sci U S A. 2010. PMID: 20498086 Free PMC article. - Dbp5, Gle1-IP6 and Nup159: a working model for mRNP export.
Folkmann AW, Noble KN, Cole CN, Wente SR. Folkmann AW, et al. Nucleus. 2011 Nov-Dec;2(6):540-8. doi: 10.4161/nucl.2.6.17881. Epub 2011 Nov 1. Nucleus. 2011. PMID: 22064466 Free PMC article. Review. - Mechanisms of nuclear mRNA export: A structural perspective.
Xie Y, Ren Y. Xie Y, et al. Traffic. 2019 Nov;20(11):829-840. doi: 10.1111/tra.12691. Epub 2019 Sep 12. Traffic. 2019. PMID: 31513326 Free PMC article. Review.
Cited by
- A checkpoint function for Nup98 in nuclear pore formation suggested by novel inhibitory nanobodies.
Solà Colom M, Fu Z, Gunkel P, Güttler T, Trakhanov S, Srinivasan V, Gregor K, Pleiner T, Görlich D. Solà Colom M, et al. EMBO J. 2024 Jun;43(11):2198-2232. doi: 10.1038/s44318-024-00081-w. Epub 2024 Apr 22. EMBO J. 2024. PMID: 38649536 Free PMC article. - Visualizing HIV-1 Capsid and Its Interactions with Antivirals and Host Factors.
Wilbourne M, Zhang P. Wilbourne M, et al. Viruses. 2021 Feb 4;13(2):246. doi: 10.3390/v13020246. Viruses. 2021. PMID: 33557422 Free PMC article. Review. - Regulation of mRNA trafficking by nuclear pore complexes.
Bonnet A, Palancade B. Bonnet A, et al. Genes (Basel). 2014 Sep 2;5(3):767-91. doi: 10.3390/genes5030767. Genes (Basel). 2014. PMID: 25184662 Free PMC article. Review. - The molecular architecture of the nuclear basket.
Singh D, Soni N, Hutchings J, Echeverria I, Shaikh F, Duquette M, Suslov S, Li Z, van Eeuwen T, Molloy K, Shi Y, Wang J, Guo Q, Chait BT, Fernandez-Martinez J, Rout MP, Sali A, Villa E. Singh D, et al. Cell. 2024 Sep 19;187(19):5267-5281.e13. doi: 10.1016/j.cell.2024.07.020. Epub 2024 Aug 9. Cell. 2024. PMID: 39127037 Free PMC article. - Structural and functional analysis of the C-terminal domain of Nup358/RanBP2.
Lin DH, Zimmermann S, Stuwe T, Stuwe E, Hoelz A. Lin DH, et al. J Mol Biol. 2013 Apr 26;425(8):1318-29. doi: 10.1016/j.jmb.2013.01.021. Epub 2013 Jan 23. J Mol Biol. 2013. PMID: 23353830 Free PMC article.
References
- Hoelz A, Debler EW, Blobel G. The structure of the nuclear pore complex. Annu. Rev. Biochem. 2011;80:613–43. - PubMed
- Debler EW, Ma Y, Seo HS, Hsia KC, Noriega TR, Blobel G, Hoelz A. A fence-like coat for the nuclear pore membrane. Mol. Cell. 2008;32:815–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous