Dimerization and direct membrane interaction of Nup53 contribute to nuclear pore complex assembly - PubMed (original) (raw)
Dimerization and direct membrane interaction of Nup53 contribute to nuclear pore complex assembly
Benjamin Vollmer et al. EMBO J. 2012.
Abstract
Nuclear pore complexes (NPCs) fuse the two membranes of the nuclear envelope (NE) to a pore, connecting cytoplasm and nucleoplasm and allowing exchange of macromolecules between these compartments. Most NPC proteins do not contain integral membrane domains and thus it is largely unclear how NPCs are embedded and anchored in the NE. Here, we show that the evolutionary conserved nuclear pore protein Nup53 binds independently of other proteins to membranes, a property that is crucial for NPC assembly and conserved between yeast and vertebrates. The vertebrate protein comprises two membrane binding sites, of which the C-terminal domain has membrane deforming capabilities, and is specifically required for de novo NPC assembly and insertion into the intact NE during interphase. Dimerization of Nup53 contributes to its membrane interaction and is crucial for its function in NPC assembly.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
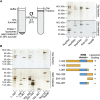
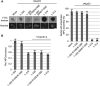
Nup53 directly binds membranes. (A) 3 μM recombinant Xenopus Nup53 (Nup53xl), the two yeast orthologues Nup59sc and Nup53sc as well as fragments of Nup133 and Nup98 as positive and negative controls, respectively, were incubated with 6 mg/ml fluorescently labelled liposomes prepared from E. coli polar lipids and floated through a sucrose gradient as indicated on the left. Top fractions of the gradient, as well as 3% of the starting material, were analysed by SDS–PAGE and silver staining. Please note that Nup53 is sensitive to C-terminal degradation (*) and that the full-length protein significantly enriched in the liposome bound fraction. (B) Full-length (1–320) and different fragments of Xenopus Nup53 as well as fragments of Nup133 and Nup98 were analysed for liposome binding as in (A). Only fragments comprising the RRM domain (indicated in blue in the schematic representation) bound liposomes.
Figure 2
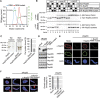
Dimerization of the RRM domain is essential for Nup53 membrane binding and nuclear pore complex formation. (A) Size exclusion chromatography on a Superdex75 10/300 GL column followed by multi-angle static laser light scattering of the Xenopus Nup53 RRM domain and the F172E/W203E mutant, which rendered the domain monomeric. The green dots relate to the secondary axis and show the molecular weight of the eluting particles. (B) HeLa cells were co-transfected with myc- and HA-tagged Xenopus Nup53 wild-type protein and/or dimerization mutant as indicated (●). Proteins were immunoprecipitated from cell lysates with α-myc or α-HA antibodies, and analysed by western blotting. Ten per cent of the start materials are loaded as input. To exclude complex formation after cell lysis, extracts from single transfected myc–Nup53 and HA–Nup53 cell batches were mixed and processed for immunoprecipitation ( ). Under these conditions, no co-precipitation was observed. (C) Full-length Xenopus Nup53 and the respective F172E/W203E mutant (RRM mutant) were analysed for liposome binding as in Figure 1. The right panel shows the quantitation of liposome binding analysed by western blotting and normalized to the levels of the wild-type protein (one out of two independent experiments). (D) Western blot analysis of mock, Nup53-depleted (ΔNup53) and Nup53-depleted extracts supplemented with recombinant wild-type protein (Nup53) or the dimerization mutant (Nup53 RRM mutant), respectively. Recombinant proteins were added to approximate endogenous Nup53 levels (judged by the full-length protein, please note for both endogenous and recombinant Nup53 proteins C-terminal degradation products (*)). The recombinant Nup53 migrated slightly faster than the endogenous protein due to absence of eukaryotic post-translational modifications. The Nup93 antibody recognizes a slightly slower migrating cross-reactivity by western blotting (*). (E) Nuclei were assembled in mock, Nup53-depleted extracts or Nup53-depleted extracts supplemented with wild-type protein (Nup53) or the dimerization mutant for 120 min, fixed with 4% PFA and analysed with Nup53 antibodies (red) and mAb414 (green). Chromatin was stained with DAPI. Bar: 10 μm. (F) Nuclei were assembled as in (E), fixed with 2% PFA and 0.5% glutaraldehyde and analysed for chromatin (blue: DAPI) and membrane staining (red: DiIC18, bar: 10 μm). Right panel shows the quantitation of chromatin substrates with a closed nuclear envelope (averages of three independent experiments with >300 randomly chosen chromatin substrates per sample, error bars represent the range).
). Under these conditions, no co-precipitation was observed. (C) Full-length Xenopus Nup53 and the respective F172E/W203E mutant (RRM mutant) were analysed for liposome binding as in Figure 1. The right panel shows the quantitation of liposome binding analysed by western blotting and normalized to the levels of the wild-type protein (one out of two independent experiments). (D) Western blot analysis of mock, Nup53-depleted (ΔNup53) and Nup53-depleted extracts supplemented with recombinant wild-type protein (Nup53) or the dimerization mutant (Nup53 RRM mutant), respectively. Recombinant proteins were added to approximate endogenous Nup53 levels (judged by the full-length protein, please note for both endogenous and recombinant Nup53 proteins C-terminal degradation products (*)). The recombinant Nup53 migrated slightly faster than the endogenous protein due to absence of eukaryotic post-translational modifications. The Nup93 antibody recognizes a slightly slower migrating cross-reactivity by western blotting (*). (E) Nuclei were assembled in mock, Nup53-depleted extracts or Nup53-depleted extracts supplemented with wild-type protein (Nup53) or the dimerization mutant for 120 min, fixed with 4% PFA and analysed with Nup53 antibodies (red) and mAb414 (green). Chromatin was stained with DAPI. Bar: 10 μm. (F) Nuclei were assembled as in (E), fixed with 2% PFA and 0.5% glutaraldehyde and analysed for chromatin (blue: DAPI) and membrane staining (red: DiIC18, bar: 10 μm). Right panel shows the quantitation of chromatin substrates with a closed nuclear envelope (averages of three independent experiments with >300 randomly chosen chromatin substrates per sample, error bars represent the range).
Figure 3
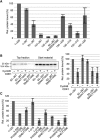
Nup53 possesses two independent membrane binding regions. (A) Full-length protein (1–320) and different fragments of Xenopus Nup53 were quantitatively analysed for liposome binding as in Figure 2B (normalized to the full-length protein, three independent experiments). (B) A fragment comprising the first membrane binding region and the RRM domain (93–267) as well as a phosphomimetic (93–267 S94E/T100E) and a non-phosphorylatable mutant (93–267 S94A/T100A) was treated with CyclinB/CDK1. Samples were tested for liposome binding as in Figure 1A and analysed by western blotting (left panel) and quantified (right panel: two independent experiments, normalized to liposome binding of wild-type fragment without CDK1 pretreatment). (C) Mutants/truncations affecting the N- (1–320 R105E/K106E, 1–320 S94E/T100E), the C-terminal (1–319, 1–312) as well as both (1–319 R105E/K106E, 1–319 S94E/T100E, 1–312 R105E/K106E, 1–312 S94E/T100E) membrane binding sites of Nup53 were quantitatively assayed for liposome binding in the context of full-length protein (normalized to wild-type protein (1–320), average of three independent experiments, error bars represent the range).
Figure 4
Either of the two membrane binding regions of Nup53 is sufficient for postmitotic NPC assembly. (A) Nuclei were assembled in mock, Nup53-depleted extracts or Nup53-depleted extracts supplemented with wild-type Nup53 (1–320), constructs featuring mutations in the N-terminal membrane binding site (1–320 R105E/K106E, 1–320 S94E/T100E), constructs lacking the C-terminal membrane binding site (1–319, 1–312) or both (1–319 R105E/K106E, 1–319 S94E/T100E, 1–312 R105E/K106E, 1–312 S94E/T100E), respectively. Samples were fixed after 120 min with 4% PFA and analysed with Nup53 antibodies (red) and mAb414 (green, upper panel) or with 2% PFA and 0.5% glutaraldehyde and analysed for chromatin (blue: DAPI) and membrane staining (red: DiIC18, lower panel). Bars: 10 μm. (B) Quantitation of chromatin substrates with a closed NE was done as in Figure 2F. (C) Western blot analysis of extracts used in (A) showing the re-addition of the recombinant proteins to approximately endogenous Nup53 levels. (D) Nuclei were assembled as in (A), fixed with 4% PFA and analysed with respective antibodies. Bar: 10 μm.
Figure 5
The C-terminal Nup53 membrane binding site is essential for interphasic nuclear pore complex (NPC) formation. (A) Nuclei were preassembled in mock or Nup53-depleted extracts supplemented with wild-type full-length protein (1–320), constructs with a mutated N-terminal membrane binding site (1–320 R105E/K106E, 1–320 S94E/T100E), or constructs lacking the C-terminal membrane interaction site (1–319, 1–312), respectively. After 90 min, the samples were supplemented with cytosol depleted of Nup53 and FG-containing nucleoporins. After 60 min, FITC-labelled 70-kDa dextran and Hoechst was added. Bar: 10 μm. The right panel shows the quantitation of three independent experiments with >300 randomly chosen chromatin substrates per sample. Error bars represent the range. (B) Nuclei were assembled in Nup53-depleted extracts supplemented with wild-type full-length protein (1–320), a construct with a mutated N-terminal membrane binding site (1–320 R105E/K106E), or a construct lacking the last C-terminal amino acid (1–319), respectively, for 120 min. Where indicated, de novo NPC assembly was blocked by the addition of 2 μM importin β after 50 min and nuclei were further incubated for 70 min. For each construct, total NPC numbers per nucleus identified by mAB414 immunofluorescence were quantified from 20 nuclei in 2 independent experiments and normalized to the wild-type full-length protein. Error bars represent the s.e. of the mean.
Figure 6
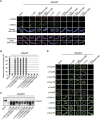
The C-terminus of Nup53 binds and deforms membranes. (A) Folch fraction I liposomes were incubated where indicated with 3 μM recombinant Nup53 fragments containing both (93–320), the N-terminal (93–267) or C-terminal (130–320) membrane binding sites including the RRM domain as well as fragments and mutants where the C-terminal (93–312, 93–319), the N-terminal (93–320 R105E/K106E, 93–320 S94E/T100E) membrane interaction site or the dimerization (93–320 RRM mutant and 130–320 RRM mutant) is compromised. The liposome deforming protein EHD2 (aa 1–543) and a fragment of Nup133 were used as positive and negative control, respectively. (B) 3 μM recombinant yeast Nup53 and Nup59 protein was incubated with liposomes and analysed. Bars: 400 nm.
Figure 7
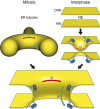
The role of Nup53 in NPC assembly. Schematic drawing of the postmitotic and interphasic modes of nuclear pore assembly focused on the membrane interacting function of Nup53. For the sake of clarity other membrane associated and integral proteins, including nucleoporins, participating in this process are omitted. Left pathway: after mitosis, outgrowing ER tubules (yellow) surround assembling NPCs providing negative/concave (red) and positive/convex (green) curvature which is stabilized by membrane binding Nup53 dimers (blue) for pore formation. Right pathway: in interphase, the intact nuclear envelope membranes (yellow) are deformed by the C-terminal membrane binding site of Nup53 introducing a convex membrane curvature for the approximation and following fusion of the two membranes leading to pore formation.
Similar articles
- Interaction of Nup53 with Ndc1 and Nup155 is required for nuclear pore complex assembly.
Eisenhardt N, Redolfi J, Antonin W. Eisenhardt N, et al. J Cell Sci. 2014 Feb 15;127(Pt 4):908-21. doi: 10.1242/jcs.141739. Epub 2013 Dec 20. J Cell Sci. 2014. PMID: 24363447 - Nup53 is required for nuclear envelope and nuclear pore complex assembly.
Hawryluk-Gara LA, Platani M, Santarella R, Wozniak RW, Mattaj IW. Hawryluk-Gara LA, et al. Mol Biol Cell. 2008 Apr;19(4):1753-62. doi: 10.1091/mbc.e07-08-0820. Epub 2008 Feb 6. Mol Biol Cell. 2008. PMID: 18256286 Free PMC article. - Nup153 Recruits the Nup107-160 Complex to the Inner Nuclear Membrane for Interphasic Nuclear Pore Complex Assembly.
Vollmer B, Lorenz M, Moreno-Andrés D, Bodenhöfer M, De Magistris P, Astrinidis SA, Schooley A, Flötenmeyer M, Leptihn S, Antonin W. Vollmer B, et al. Dev Cell. 2015 Jun 22;33(6):717-28. doi: 10.1016/j.devcel.2015.04.027. Epub 2015 Jun 4. Dev Cell. 2015. PMID: 26051542 - Dunking into the Lipid Bilayer: How Direct Membrane Binding of Nucleoporins Can Contribute to Nuclear Pore Complex Structure and Assembly.
Hamed M, Antonin W. Hamed M, et al. Cells. 2021 Dec 20;10(12):3601. doi: 10.3390/cells10123601. Cells. 2021. PMID: 34944108 Free PMC article. Review. - Nuclear envelope insertion of spindle pole bodies and nuclear pore complexes.
Jaspersen SL, Ghosh S. Jaspersen SL, et al. Nucleus. 2012 May-Jun;3(3):226-36. doi: 10.4161/nucl.20148. Epub 2012 May 1. Nucleus. 2012. PMID: 22572959 Free PMC article. Review.
Cited by
- A model for coordinating nuclear mechanics and membrane remodeling to support nuclear integrity.
King MC, Lusk CP. King MC, et al. Curr Opin Cell Biol. 2016 Aug;41:9-17. doi: 10.1016/j.ceb.2016.03.009. Epub 2016 Mar 28. Curr Opin Cell Biol. 2016. PMID: 27031045 Free PMC article. Review. - Nuclear pore complex integrity requires Lnp1, a regulator of cortical endoplasmic reticulum.
Casey AK, Chen S, Novick P, Ferro-Novick S, Wente SR. Casey AK, et al. Mol Biol Cell. 2015 Aug 1;26(15):2833-44. doi: 10.1091/mbc.E15-01-0053. Epub 2015 Jun 3. Mol Biol Cell. 2015. PMID: 26041935 Free PMC article. - Generating Membrane Curvature at the Nuclear Pore: A Lipid Point of View.
Peeters BWA, Piët ACA, Fornerod M. Peeters BWA, et al. Cells. 2022 Jan 29;11(3):469. doi: 10.3390/cells11030469. Cells. 2022. PMID: 35159279 Free PMC article. Review. - Lipid Droplets Are a Physiological Nucleoporin Reservoir.
Kumanski S, Viart BT, Kossida S, Moriel-Carretero M. Kumanski S, et al. Cells. 2021 Feb 22;10(2):472. doi: 10.3390/cells10020472. Cells. 2021. PMID: 33671805 Free PMC article. - Melatonin: Regulation of Biomolecular Condensates in Neurodegenerative Disorders.
Loh D, Reiter RJ. Loh D, et al. Antioxidants (Basel). 2021 Sep 17;10(9):1483. doi: 10.3390/antiox10091483. Antioxidants (Basel). 2021. PMID: 34573116 Free PMC article. Review.
References
- Amlacher S, Sarges P, Flemming D, van Noort V, Kunze R, Devos DP, Arumugam M, Bork P, Hurt E (2011) Insight into structure and assembly of the nuclear pore complex by utilizing the genome of a eukaryotic thermophile. Cell 146: 277–289 - PubMed
- Antonin W, Ellenberg J, Dultz E (2008) Nuclear pore complex assembly through the cell cycle: regulation and membrane organization. FEBS Lett 582: 2004–2016 - PubMed
- Antonin W, Franz C, Haselmann U, Antony C, Mattaj IW (2005) The integral membrane nucleoporin pom121 functionally links nuclear pore complex assembly and nuclear envelope formation. Mol Cell 17: 83–92 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials