MiR-214 targets β-catenin pathway to suppress invasion, stem-like traits and recurrence of human hepatocellular carcinoma - PubMed (original) (raw)
MiR-214 targets β-catenin pathway to suppress invasion, stem-like traits and recurrence of human hepatocellular carcinoma
Hongping Xia et al. PLoS One. 2012.
Erratum in
- PLoS One. 2012;7(9). doi:10.1371/annotation/1be2a62e-45a1-4c13-9a8d-f265005a21e0
Abstract
The down-regulation of miR-214 has previously been observed in human hepatocellular carcinoma (HCC). Here, we demonstrated the down-regulation of miR-214 is associated with cell invasion, stem-like traits and early recurrence of HCC. Firstly, we validated the suppression of miR-214 in human HCC by real-time quantitative RT-PCR (qRT-PCR) in 20 paired tumor and non-tumor liver tissues of HCC patients and 10 histologically normal liver tissues from colorectal cancer patients with liver metastases. Further qRT-PCR analysis of 50 HCC tissues from an independent cohort of HCC patients of whom 29 with early recurrent disease (<2 years) and 21 with late recurrent disease demonstrated that the suppression of miR-214 was significantly more suppressed in samples from HCC patients with early recurrent disease compared those from patients with no recurrence. Re-expression of miR-214 significantly suppressed the growth of HCC cells in vitro and reduced their tumorigenicity in vivo. The enhancer of zeste homologue 2 (EZH2) and β-catenin (CTNNB1) was identified as two potential direct downstream targets of miR-214 through bioinformatics analysis and experimentally validated the miRNA-target interactions with a dual-firefly luciferase reporter assay. In corroborate with this, both EZH2 and CTNNB1 are found to be significantly overexpressed in human HCC biopsies. Since EZH2 can regulate CTNNB1, CTNNB1 can also be an indirect target of miR-214 through EZH2. Silencing EZH2 or CTNNB1 expression suppressed the growth and invasion of HCC cells and induced E-cadherin (CDH1), known to inhibit cell invasion and metastasis. Furthermore, the silencing of miR-214 or overexpression of EZH2 increased EpCAM(+) stem-like cells through the activation of CTNNB1. Interestingly, the up-regulation of EZH2, CTNNB1 and the down-regulation of CDH1 in HCC patients correlated with early recurrent disease and can be an independent predictor of poor survival. Therefore, miR-214 can directly or indirectly target CTNNB1 to modulate the β-catenin signaling pathway in HCC.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
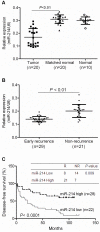
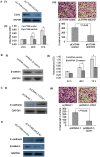
Figure 1. Downregulation of miR-214 is associated with the early recurrence of human HCC.
The level of expression of miR-214 was analyzed by qRT-PCR and normalized to U6. (A) Expression of miR-214 in 20 paired of HCC tumor tissues was significantly lower compared to 20 matched histologically normal tissues as well as 10 histologically normal liver tissues from colorectal cancer patients with liver metastases (P<0.01). (B) Low miR-214 expression was associated with early recurrent HCC disease when studied in an independent cohort of 50 HCC samples. The average expression level of miR-214, analyzed by qRT-PCR, was lower in HCC patients with early recurrence (≤2 years) (n = 29) than patients with no recurrence in the same time period (n = 21). (C) The expression of miR-214 was associated with survival in patients with HCC. The median expression value obtained for miR-214 of the 50 samples studied was employed as the cut-off point. Fisher’s exact test and Kaplan-Meier analysis were used to demonstrate that high miR-214 expression was significantly associated with early recurrent disease and a relative poorer disease-free survival rate.
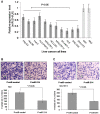
Figure 2. MiR-214 inhibits the invasion of HCC cells.
(A) The expression of miR-214 in liver tumor cell lines was significantly lower than in the normal liver tissues (NN1 and NN2) (* P<0.05). (B and C) Re-expression of miR-214 following transfection with P-miR-214 inhibited the invasion of HLE (B) and SK-HEP-1 (C) cells. The upper panels in the figures showed images of transwell migration. The bar graph below the images indicated the mean number of invaded cells (± SD) counted under the microscope in three randomly selected fields (magnification ×40). *P<0.05.
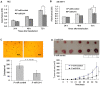
Figure 3. Re-expression of miR-214 significantly inhibits cell growth in vitro and tumorigenic properties of HCC cells.
(A and B) Growth of HLE (A) and SK-HEP-1 (B) cells in vitro at different time points following the re-expression of miR-214 mediated by transfection with P-miR-214, *P<0.05. (C) Stable expression of miR-214 inhibited the anchorage-independent growth of SK-HEP-1 cells in soft agar. The upper section shows images of colony formation. The bar graph below the figures showed the mean number of colonies formed (± SD) and counted under the microscope in three randomly selected fields (magnification, ×40). *P<0.05. (D) Stable expression of miR-214 inhibited tumorigenicity of SK-HEP-1 cells. The upper section showed images of the tumors obtained in mice at the end of the eighth week. The bar graph indicated the average of overall tumor volume measured each week (n = 6 mice per group).
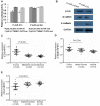
Figure 4. EZH2 and CTNNB1 are downstream targets of miR-214 and both are upregulated in human HCC tissue samples.
(A) Effect of miR-214 on EZH2 and CTNNB1 expression, as shown by a luciferase reporter assay. The data were normalized by the ratio of Firefly and Renilla luciferase activities measured at 48 h post-transfection. The bar graph showed the mean ± SD in three independent transfection experiments. *P<0.05. (B) Western blotting analysis of EZH2, β-catenin, and E-cadherin expression in P-miR-control- and P-miR-214-transfected SK-HEP-1 cells. (C-E) Validation of the expression of EZH2 (C), CTNNB1 (D) and CDH1 (E) in 20 paired human HCC tissue samples and 10 samples of histologically normal liver tissues were validated by qRT-PCR.
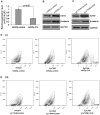
Figure 5. Roles of EZH2, CTNNB1 and CDH1 on the growth and invasion of HCC cells.
(A) Silencing of EZH2 significantly inhibited the growth and significant decreased the ability of SK-HEP-1 cells to invade. (i) Western blots showing the silencing of EZH2 by pLVTHM-shEZH2. (ii) The effect of silencing EZH2 on cell growth at different time points. (iii) The inhibitory effect of silencing EZH2 on cell invasion. (B) Silencing of CTNNB1 significantly inhibited the growth of SK-HEP-1 cells. (i) Western blots showing the reduction of β-catenin after transfection with shRNA-β-catenin. (ii) Effects of silencing β-catenin on cell growth at different time points. (C) Over-expression CDH1 significantly inhibited the ability of SK-HEP-1 cells to invade. (i) Western blots showing the overexpression of CDH1 by pcDNA3.1-CDH1 plasmid transfection. (ii) The inhibitory effects of overexpressing CDH1 on SK-HEP-1 cell invasion. (D) Western blots showing the silencing of EZH2 significantly decreased the expression of CTNNB1 and induced CDH1 expression.
Figure 6. Further silencing of miR-214 in HLE cells with miRZip-214 increases EpCAM+ stem-like cell population by activating the β-catenin pathway.
(A) Further silencing of miR-214 in HLE cells with miRZip-214. (B) Silencing miR-214 following transfection with miRZip-214 increased EZH2 and β-catenin expression. (C) Overexpression of EZH2 with pLVTHM-EZH2 activated β-catenin expression. (D) Flow cytometric analysis of EpCAM+ stem-like HLE cells following the silencing of miR-214 (i) and overexpression of EZH2 (ii).
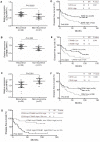
Figure 7. Expression of EZH2, CTNNB1, and CDH1, downstream targets of miR-214, is associated with early recurrent disease and survival of patients with HCC.
The level of expression of EZH2, CTNNB1, and CDH1 in the 50 HCC samples described in Figure 1 was analyzed by qRT-PCR and normalized by HPRT1. (A–C) The average expression level of EZH2 and CTNNB1 was significantly higher in samples from HCC patients with early recurrence (≤2 years) (n = 29) than patients with no recurrence over the same period (n = 21). In comparison, the average expression level of CDH1 was significantly lower in in samples from HCC patients with early recurrence (C). (D–F) The median expression value obtained for EZH2, CTNNB1, and CDH1 of the 50 samples studied was employed as the cut-off point and employed independently in Kaplan-Meier analysis to study disease-free survival rate. (G) Combining the expression of EZH2, CTNNB1 and CDH1 to predict tumor recurrence and disease-free survival rate using Kaplan-Meier analysis.
Similar articles
- MicroRNA-26a suppresses epithelial-mesenchymal transition in human hepatocellular carcinoma by repressing enhancer of zeste homolog 2.
Ma DN, Chai ZT, Zhu XD, Zhang N, Zhan DH, Ye BG, Wang CH, Qin CD, Zhao YM, Zhu WP, Cao MQ, Gao DM, Sun HC, Tang ZY. Ma DN, et al. J Hematol Oncol. 2016 Jan 6;9:1. doi: 10.1186/s13045-015-0229-y. J Hematol Oncol. 2016. PMID: 26733151 Free PMC article. - MicroRNA-101 inhibits human hepatocellular carcinoma progression through EZH2 downregulation and increased cytostatic drug sensitivity.
Xu L, Beckebaum S, Iacob S, Wu G, Kaiser GM, Radtke A, Liu C, Kabar I, Schmidt HH, Zhang X, Lu M, Cicinnati VR. Xu L, et al. J Hepatol. 2014 Mar;60(3):590-8. doi: 10.1016/j.jhep.2013.10.028. Epub 2013 Nov 6. J Hepatol. 2014. PMID: 24211739 - PRRX1 deficiency induces mesenchymal-epithelial transition through PITX2/miR-200-dependent SLUG/CTNNB1 regulation in hepatocellular carcinoma.
Chen W, Wu J, Shi W, Zhang G, Chen X, Ji A, Wang Z, Wu J, Jiang C. Chen W, et al. Cancer Sci. 2021 Jun;112(6):2158-2172. doi: 10.1111/cas.14853. Epub 2021 Apr 8. Cancer Sci. 2021. PMID: 33587761 Free PMC article. - Epigenetic regulation of cancer stem cells in liver cancer: current concepts and clinical implications.
Marquardt JU, Factor VM, Thorgeirsson SS. Marquardt JU, et al. J Hepatol. 2010 Sep;53(3):568-77. doi: 10.1016/j.jhep.2010.05.003. Epub 2010 May 31. J Hepatol. 2010. PMID: 20646772 Free PMC article. Review. - MicroRNAs and liver cancer associated with iron overload: therapeutic targets unravelled.
Greene CM, Varley RB, Lawless MW. Greene CM, et al. World J Gastroenterol. 2013 Aug 28;19(32):5212-26. doi: 10.3748/wjg.v19.i32.5212. World J Gastroenterol. 2013. PMID: 23983424 Free PMC article. Review.
Cited by
- Crosstalk between the Hippo Pathway and the Wnt Pathway in Huntington's Disease and Other Neurodegenerative Disorders.
Sileo P, Simonin C, Melnyk P, Chartier-Harlin MC, Cotelle P. Sileo P, et al. Cells. 2022 Nov 16;11(22):3631. doi: 10.3390/cells11223631. Cells. 2022. PMID: 36429058 Free PMC article. Review. - Recent insights into hepatic cancer stem cells.
Anfuso B, Tiribelli C, Sukowati CH. Anfuso B, et al. Hepatol Int. 2014 Sep;8 Suppl 2:458-63. doi: 10.1007/s12072-013-9498-0. Epub 2013 Dec 20. Hepatol Int. 2014. PMID: 26201324 - Epigenetic Activation of Wnt/β-Catenin Signaling in NAFLD-Associated Hepatocarcinogenesis.
Tian Y, Mok MT, Yang P, Cheng AS. Tian Y, et al. Cancers (Basel). 2016 Aug 20;8(8):76. doi: 10.3390/cancers8080076. Cancers (Basel). 2016. PMID: 27556491 Free PMC article. Review. - miRNA and TMPRSS2-ERG do not mind their own business in prostate cancer cells.
Fayyaz S, Farooqi AA. Fayyaz S, et al. Immunogenetics. 2013 May;65(5):315-32. doi: 10.1007/s00251-012-0677-2. Epub 2013 Apr 5. Immunogenetics. 2013. PMID: 23558555 Review. - MicroRNA-143 inhibits tumor growth and angiogenesis and sensitizes chemosensitivity to oxaliplatin in colorectal cancers.
Qian X, Yu J, Yin Y, He J, Wang L, Li Q, Zhang LQ, Li CY, Shi ZM, Xu Q, Li W, Lai LH, Liu LZ, Jiang BH. Qian X, et al. Cell Cycle. 2013 May 1;12(9):1385-94. doi: 10.4161/cc.24477. Epub 2013 Apr 8. Cell Cycle. 2013. PMID: 23574723 Free PMC article.
References
- Sanyal AJ, Yoon SK, Lencioni R (2010) The Etiology of Hepatocellular Carcinoma and Consequences for Treatment. The Oncologist 15: 14–22. - PubMed
- Jemal A, Bray F (2011) Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. - PubMed
- Pei Y, Zhang T, Renault V, Zhang X (2009) An overview of hepatocellular carcinoma study by omics-based methods. Acta Biochim Biophys Sin 41: 1–15. - PubMed
- Wang SM, Ooi LLPJ, Hui KM (2011) Upregulation of Rac GTPase-Activating Protein 1 Is Significantly Associated with the Early Recurrence of Human Hepatocellular Carcinoma. Clin Cancer Res 17: 6040–6051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by by grants from the National Medical Research Council, Biomedical Research Council of Singapore and The Singapore Millennium Foundation, http://www.nmrc.gov.sg/content/nmrc_internet/home.html. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous