Secretion of soluble vascular endothelial growth factor receptor 1 (sVEGFR1/sFlt1) requires Arf1, Arf6, and Rab11 GTPases - PubMed (original) (raw)
Secretion of soluble vascular endothelial growth factor receptor 1 (sVEGFR1/sFlt1) requires Arf1, Arf6, and Rab11 GTPases
Jae-Joon Jung et al. PLoS One. 2012.
Erratum in
- PLoS One. 2012;7(11). doi:.1371/annotation/19ed28f9-9a01-4af9-857d-3ec4aaeceea1
Abstract
The soluble form of vascular endothelial growth factor receptor 1 (sVEGFR-1/sFlt1) is generated by alternative splicing of the FLT1 gene. Secretion of sFlt1 from endothelial cells plays an important role in blood vessel sprouting and morphogenesis. However, excess sFlt1 secretion is associated with diseases such as preeclampsia and chronic kidney disease. To date, the secretory transport process involved in the secretion of sFlt1 is poorly understood. In the present study, we investigated the itinerary of sFlt1 trafficking along the secretory pathway. To understand the timecourse of sFlt1 secretion, endothelial cells stably expressing sFlt1 were metabolically radiolabeled with [(35)S]-methionine and cysteine. Our results indicate that after initial synthesis the levels of secreted [(35)S]-sFlt1 in the extracellular medium peaks at 8 hours. Treatment with brefeldin A (BFA), a drug which blocks trafficking between the endoplasmic reticulum (ER) and the Golgi complex, inhibited extracellular release of sFlt1 suggesting that ER to Golgi and intra-Golgi trafficking of sFlt1 are essential for its secretion. Furthermore, we show that ectopic expression of dominant-negative mutant forms of Arf1, Arf6, and Rab11 as well as siRNA-mediated knockdown of these GTPases block secretion of sFlt1 during normoxic and hypoxic conditions suggesting role for these small GTPases. This work is the first to report role of regulatory proteins involved in sFlt1 trafficking along the secretory pathway and may provide insights and new molecular targets for the modulation of sFlt-1 release during physiological and pathological conditions.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
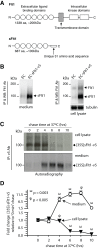
Figure 1. A: Schematic representation of domain organization of full-length Flt1 and soluble Flt1 (sFlt1).
B: HUVECs stably expressing v5-tagged sFlt1 (EC-sFlt1) and HUVEC control cells were seeded at 30% confluency and cultured for 5 days before collection of spent culture media and preparation of cell lysates. Immunoprecipitation (IP) of endogenous sFlt1 and ectopic sFlt1-v5 from cell lysates and spent culture supernatants was performed using an Ab to Flt1. Blot shows robust cellular expression and secretion of sFlt1-v5 in EC-sFlt1 stable cells relative to control HUVECs (EC). C: Time course of sFlt1-v5 secretion after synthesis. EC-sFlt1 cells (80% confluency) were metabolically labeled with 35S-methionine-35S-cysteine for 20 min. After the indicated chase period in an excess of unlabeled methionine-cysteine, cells were lysed, and spent culture media and cell lysates were TCA precipitated. Radioactive counts were normalized prior to IP using an anti-v5 Ab. The immunoprecipitated samples were analyzed by SDS-PAGE; gels were dried and exposed for autoradiography. D: Autoradiographic signals from films were measured using Image J (NIH) software and are presented as fold change relative to the 0 min chase values. Values are mean ± SEM; n = 3.
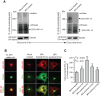
Figure 2. Brefeldin A (BFA) treatment blocks sFlt1 secretion and increases intracellular accumulation.
A: EC-sFlt1 cells were either mock treated or pretreated with BFA (1 μg/ml) for 20 min before metabolically labeling with 35S-methionine-35S-cysteine for 20 min. Samples were then chased for 6 hrs in excess of unlabeled methionine-cysteine containing medium without (in control) or with BFA (1 μg/ml). Cells were then lysed and spent culture supernatant and cell lysates were TCA precipitated. Radioactive counts were normalized prior to immunoprecipitation with anti-v5 Ab. The immunoprecipitated samples were analyzed by SDS-PAGE, gels were dried and exposed for autoradiography, and representative autoradiographs are shown. B: EC-sFlt1 stable cells were grown in medium without (mock treated) or with BFA (1 μg/ml) for either 2 hrs or 6 hrs. A set of samples, after 6 h of BFA treatment, was subjected to BFA washout for 2 hrs or 4 hrs. Cells were fixed, permeabilized, and labeled with Abs against v5, Flt1, or TGN46 (Golgi marker) followed by the appropriate fluorescently-tagged secondary Ab. Representative images obtained by epifluorescence microscopy show localization of v5-tagged sFlt1. C: Quantification of intracellular retention of v5-sFlt1 upon BFA treatment and BFA washout steps. Epifluorescence images were acquired and total cell-associated fluorescence was quantified by image analysis. Values represent relative change in the levels of v5-sFlt1 normalized to an arbitrary value of 100% for untreated controls. Results are expressed as mean ± SEM (n = 70 cells for each condition, from 3 separate experiments). Scale bar represents 5 µm.
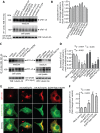
Figure 3. Secretion of sFlt1 from endothelial cells requires functional Arf6, Arf1, and Rab11 GTPases.
A–F: EC-sFlt1 cells were nucleofected with plasmid constructs expressing dominant-negative forms of Arf1, Arf6, Rab5, Rab6, Rab 8, syntaxin 4, syntaxin 6, and syntaxin 16. Similarly, in a separate study EC-sFlt1 cells were nucleofected with scramble oligonucleotide or siRNAs against Arf1, Arf6, and Rab11a. Media containing nucleofection complexes were replaced with fresh media 8 hrs post-nucleofection. At 36 hrs post-nucleofection, secreted and intracellular sFlt1 were immunoprecipitated from spent culture media and cell lysates using the anti-v5 Ab. (A, C) Representative blot shows relative expression of sFlt1-v5 in cell lysates and secreted in culture media. B , D: Band densities from experiments as in A, C were quantified by densitometric analysis. Secretion of sFlt1-v5 was calculated as the ratio of desitometric band intensity values of secreted to intracellular Flt1; this ratio was set at 1 for mock-transfected control EC-sFlt1 cells. The data are the means ± SEM from three independent experiments. E: EC-sFlt1 cells were transiently transfected with indicated dominant-negative plasmid constructs. After 24 hrs cells were fixed, permeabilized, and labeled with anti-v5 Ab to detect cell-associated sFlt1-v5. Representative images obtained by epifluorescence microscopy show localization of sFlt1-v5. F: Quantification of intracellular retention of sFlt1-v5 upon inhibition of Arf1, Arf6, and Rab11 function. Total cell-associated fluorescence was quantified by analysis of epifluorescence images obtained as in E. Values represent relative change in the levels of sFlt1-v5 normalized to an arbitrary value of 100% for mock-transfected control. Results are expressed as mean ± SEM (n = 70 cells for each condition, from 3 separate experiments). Scale bar represents 5 µm.
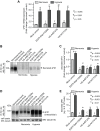
Figure 4. Role of Rab11, Arf1, and Arf6 GTPases in the secretion of sFlt1 in JEG3 cells during normoxic and hypoxic culture conditions.
JEG-3 cells were nucleofected with plasmid constructs expressing dominant negative forms of Arf1 (HA-tagged-Arf1T31N), Arf6 (HA-tagged-Arf6T27N), and Rab11 (EGFP-Rab11S25N). The spent media was replaced with fresh media after 12 hrs of nucleofection. The cells were incubated for additional 36 hrs under normoxic (8% O2) or hypoxic (2% O2) culture conditions before analyzing sFlt1 mRNA and protein levels. A : Quantitative RT-PCR analysis showing sFlt1 transcript levels relative to normoxia control. Data = mean ± SEM (n = 3). B : Spent culture media were prepared for immunoprecipitation with Flt1 Ab. Blot shows relative levels of secreted sFlt1 in the culture medium. C : Band densities from experiments as in B were quantified by densitometric analysis. D : After collecting the spent culture media for analysis, the remaining cells were lysed and a relative level of sFlt1 was determined by western blot (WB) analysis. E: Experiment was performed as in B ; amount of sFlt1 in spent culture supernatant was measured by sandwich ELISA. Values in C , E are expressed as mean ± SEM (n = 70 cells for each condition, from 3 separate experiments).
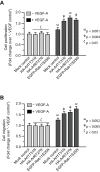
Figure 5. Inhibition of Rab11, Arf1, and Arf6 function enhances biological activity of VEGF-A as revealed by cell proliferation and migration assays.
A, B: EC-sFlt1 cells were either mock treated (control) or were nucleofected with dominant-negative forms of Arf1 (HA-tagged-Arf1T31N), Arf6 (HA-tagged-Arf6T27N), and Rab11 (EGFP-Rab11S25N). Cells were harvested at 36 hrs post-transfection for subsequent assays. A : Samples were serum-starved and then treated with VEGF-A (5 ng/ml) for 24 hrs. Cell proliferation assays were carried out using the MTT assay. Cell proliferation values were normalized to that of unstimulated, mock-transfected controls. B : Directional migration of cells toward VEGF-A (5 ng/ml) by Boyden chamber assay, with VEGF present in the lower well. The number of migrating cells was normalized to that in unstimulated, mock-transfected controls. Values represent mean ± SEM (n = 3).
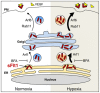
Figure 6. Schematic diagram of sFlt1 secretion and its secretory transport itinerary.
A tentative model summarizing our current view. After its initial synthesis, sFlt1 is transported from the ER to the Golgi complex; this step is sensitive to BFA treatment and also requires Arf1 GTPase. Soluble Flt1 (sFlt1) transport from the Golgi complex for subsequent secretion requires Rab11 and Arf6 GTPases. Our data predict that sFlt1 secretion is dependent on Arf1-, Arf6-, and Rab11-regulated intracellular vesicular trafficking events along the secretory pathway.
Similar articles
- Brefeldin A and M-COPA block the export of RTKs from the endoplasmic reticulum via simultaneous inactivation of ARF1, ARF4, and ARF5.
Natsume M, Niwa M, Ichikawa S, Okamoto T, Tsutsui H, Usukura D, Murata T, Abe R, Shimonaka M, Nishida T, Shiina I, Obata Y. Natsume M, et al. J Biol Chem. 2024 Jun;300(6):107327. doi: 10.1016/j.jbc.2024.107327. Epub 2024 Apr 26. J Biol Chem. 2024. PMID: 38679330 Free PMC article. - The role of ARF1 and rab GTPases in polarization of the Golgi stack.
Bannykh SI, Plutner H, Matteson J, Balch WE. Bannykh SI, et al. Traffic. 2005 Sep;6(9):803-19. doi: 10.1111/j.1600-0854.2005.00319.x. Traffic. 2005. PMID: 16101683 - A defined clathrin-mediated trafficking pathway regulates sFLT1/VEGFR1 secretion from endothelial cells.
Kinghorn K, Gill A, Marvin A, Li R, Quigley K, Singh S, Gore MT, le Noble F, Gabhann FM, Bautch VL. Kinghorn K, et al. Angiogenesis. 2024 Feb;27(1):67-89. doi: 10.1007/s10456-023-09893-6. Epub 2023 Sep 11. Angiogenesis. 2024. PMID: 37695358 Free PMC article. - ARF1 and SAR1 GTPases in endomembrane trafficking in plants.
Cevher-Keskin B. Cevher-Keskin B. Int J Mol Sci. 2013 Sep 5;14(9):18181-99. doi: 10.3390/ijms140918181. Int J Mol Sci. 2013. PMID: 24013371 Free PMC article. Review. - Control of cystic fibrosis transmembrane conductance regulator membrane trafficking: not just from the endoplasmic reticulum to the Golgi.
Farinha CM, Matos P, Amaral MD. Farinha CM, et al. FEBS J. 2013 Sep;280(18):4396-406. doi: 10.1111/febs.12392. Epub 2013 Jul 5. FEBS J. 2013. PMID: 23773658 Review.
Cited by
- Galectin-1 and -3 in high amounts inhibit angiogenic properties of human retinal microvascular endothelial cells in vitro.
Hillenmayer A, Wertheimer CM, Geerlof A, Eibl KH, Priglinger S, Priglinger C, Ohlmann A. Hillenmayer A, et al. PLoS One. 2022 Mar 23;17(3):e0265805. doi: 10.1371/journal.pone.0265805. eCollection 2022. PLoS One. 2022. PMID: 35320287 Free PMC article. - Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis.
Salomon C, Ryan J, Sobrevia L, Kobayashi M, Ashman K, Mitchell M, Rice GE. Salomon C, et al. PLoS One. 2013 Jul 8;8(7):e68451. doi: 10.1371/journal.pone.0068451. Print 2013. PLoS One. 2013. PMID: 23861904 Free PMC article. - A defined clathrin-mediated trafficking pathway regulates sFLT1/VEGFR1 secretion from endothelial cells.
Kinghorn K, Gill A, Marvin A, Li R, Quigley K, le Noble F, Mac Gabhann F, Bautch VL. Kinghorn K, et al. bioRxiv [Preprint]. 2023 Jan 28:2023.01.27.525517. doi: 10.1101/2023.01.27.525517. bioRxiv. 2023. PMID: 36747809 Free PMC article. Updated. Preprint. - Overexpression of preeclampsia induced microRNA-26a-5p leads to proteinuria in zebrafish.
Müller-Deile J, Schröder P, Beverly-Staggs L, Hiss R, Fiedler J, Nyström J, Thum T, Haller H, Schiffer M. Müller-Deile J, et al. Sci Rep. 2018 Feb 26;8(1):3621. doi: 10.1038/s41598-018-22070-w. Sci Rep. 2018. PMID: 29483572 Free PMC article. - The myosin motor Myo1c is required for VEGFR2 delivery to the cell surface and for angiogenic signaling.
Tiwari A, Jung JJ, Inamdar SM, Nihalani D, Choudhury A. Tiwari A, et al. Am J Physiol Heart Circ Physiol. 2013 Mar 1;304(5):H687-96. doi: 10.1152/ajpheart.00744.2012. Epub 2012 Dec 21. Am J Physiol Heart Circ Physiol. 2013. PMID: 23262137 Free PMC article.
References
- Kendall RL, Wang G, Thomas KA (1996) Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun 226: 324–328. - PubMed
- Park JE, Chen HH, Winer J, Houck KA, Ferrara N (1994) Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem 269: 25646–25654. - PubMed
- Roeckl W, Hecht D, Sztajer H, Waltenberger J, Yayon A, et al. (1998) Differential binding characteristics and cellular inhibition by soluble VEGF receptors 1 and 2. Exp Cell Res 241: 161–170. - PubMed
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, et al. (2004) Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01DK090053/DK/NIDDK NIH HHS/United States
- R01 DK090053/DK/NIDDK NIH HHS/United States
- R01 HL089599/HL/NHLBI NIH HHS/United States
- R01CA127958/CA/NCI NIH HHS/United States
- HL089599/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous