Treatment with sunitinib for patients with progressive metastatic pheochromocytomas and sympathetic paragangliomas - PubMed (original) (raw)
. 2012 Nov;97(11):4040-50.
doi: 10.1210/jc.2012-2356. Epub 2012 Sep 10.
Cecile N Chougnet, Mouhammed Amir Habra, J Lynn Palmer, Sophie Leboulleux, Maria E Cabanillas, Caroline Caramella, Pete Anderson, Abir Al Ghuzlan, Steven G Waguespack, Desirée Deandreis, Eric Baudin, Camilo Jimenez
Affiliations
- PMID: 22965939
- PMCID: PMC3683800
- DOI: 10.1210/jc.2012-2356
Treatment with sunitinib for patients with progressive metastatic pheochromocytomas and sympathetic paragangliomas
Montserrat Ayala-Ramirez et al. J Clin Endocrinol Metab. 2012 Nov.
Abstract
Context: Patients with progressive metastatic pheochromocytomas (PHEOs) or sympathetic paragangliomas (SPGLs) face a dismal prognosis. Current systemic therapies are limited.
Objectives: The primary end point was progression-free survival determined by RECIST 1.1 criteria or positron emission tomography with [(18)F]fluorodeoxyglucose/computed tomography ([(18)F]FDG-PET/CT), in the absence of measurable soft tissue targets. Secondary endpoints were tumor response according to RECIST criteria version 1.1 or FDG uptake, blood pressure control, and safety.
Design: We conducted a retrospective review of medical records of patients with metastatic PHEO/SPGL treated with sunitinib from December 2007 through December 2011. An intention-to-treat analysis was performed.
Patients and setting: Seventeen patients with progressive metastatic PHEO/SPGLs treated at the Institut Gustave-Roussy and MD Anderson Cancer Center.
Interventions: Patients treated with sunitinib.
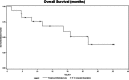
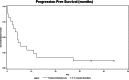
Results: According to RECIST 1.1, eight patients experienced clinical benefit; three experienced partial response, and five had stable disease, including four with predominant skeletal metastases that showed a 30% or greater reduction in glucose uptake on [(18)F]FDG-PET/CT. Of 14 patients who had hypertension, six became normotensive and two discontinued antihypertensives. One patient treated with sunitinib and rapamycin experienced a durable benefit beyond 36 months. The median overall survival from the time sunitinib was initiated was 26.7 months with a progression-free survival of 4.1 months (95% confidence interval = 1.4-11.0). Most patients who experienced a clinical benefit were carriers of SDHB mutations.
Conclusion: Sunitinib is associated with tumor size reduction, decreased [(18)F]FDG-PET/CT uptake, disease stabilization, and hypertension improvement in some patients with progressive metastatic PHEO/PGL. Prospective multi-institutional clinical trials are needed to determine the true benefits of sunitinib.
Figures
Fig. 1.
Median overall survival from sunitinib initiation.
Fig. 2.
PFS in patients with progressive metastatic PHEO or SPGL treated with sunitinib.
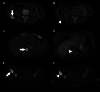
Fig. 3.
Positive response to sunitinib and rapamycin. A patient with both bone and soft-tissue sites of metastatic disease was treated with sunitinib. The results of two FDG-PET/CT studies are shown, obtained 36 months apart. A and B, A soft-tissue metastasis in the right flank decreased in intensity from an SUV of 17.5 before treatment to 2.7 after treatment; C and D, a lytic bone metastasis in the T11 vertebral body decreased in intensity from an SUV of 21.6 to 2.1; E and F, a lytic bone metastasis in the right iliac bone, although persistently hypermetabolic, showed positive response with a decline in SUV from 37.6–14.6.
Similar articles
- Sunitinib in the therapy of malignant paragangliomas: report on the efficacy in a SDHB mutation carrier and review of the literature.
Canu L, Pradella S, Rapizzi E, Fucci R, Valeri A, Briganti V, Giachè V, Parenti G, Ercolino T, Mannelli M. Canu L, et al. Arch Endocrinol Metab. 2017 Jan-Feb;61(1):90-97. doi: 10.1590/2359-3997000000217. Epub 2016 Oct 10. Arch Endocrinol Metab. 2017. PMID: 27737332 Free PMC article. Review. - Rationale and evidence for sunitinib in the treatment of malignant paraganglioma/pheochromocytoma.
Joshua AM, Ezzat S, Asa SL, Evans A, Broom R, Freeman M, Knox JJ. Joshua AM, et al. J Clin Endocrinol Metab. 2009 Jan;94(1):5-9. doi: 10.1210/jc.2008-1836. Epub 2008 Nov 11. J Clin Endocrinol Metab. 2009. PMID: 19001511 - Interferon-alpha Treatment for Disease Control in Metastatic Pheochromocytoma/Paraganglioma Patients.
Hadoux J, Terroir M, Leboulleux S, Deschamps F, Al Ghuzlan A, Hescot S, Tselikas L, Borget I, Caramella C, Déandréis D, Goere D, De Baere T, Schlumberger M, Baudin E. Hadoux J, et al. Horm Cancer. 2017 Dec;8(5-6):330-337. doi: 10.1007/s12672-017-0303-8. Epub 2017 Jul 26. Horm Cancer. 2017. PMID: 28748315 Free PMC article. - Combined positron emission tomography/computed tomography in sunitinib therapy assessment of patients with metastatic renal cell carcinoma.
Revheim ME, Winge-Main AK, Hagen G, Fjeld JG, Fosså SD, Lilleby W. Revheim ME, et al. Clin Oncol (R Coll Radiol). 2011 Jun;23(5):339-43. doi: 10.1016/j.clon.2010.11.006. Epub 2010 Dec 4. Clin Oncol (R Coll Radiol). 2011. PMID: 21134733 - Metastatic pheochromocytoma and paraganglioma.
Angelousi A, Kassi E, Zografos G, Kaltsas G. Angelousi A, et al. Eur J Clin Invest. 2015 Sep;45(9):986-97. doi: 10.1111/eci.12495. Eur J Clin Invest. 2015. PMID: 26183460 Review.
Cited by
- Diagnostic features and therapeutic strategies for malignant paraganglioma in a patient: A case report.
Gan L, Shen XD, Ren Y, Cui HX, Zhuang ZX. Gan L, et al. World J Clin Cases. 2022 Sep 26;10(27):9834-9844. doi: 10.12998/wjcc.v10.i27.9834. World J Clin Cases. 2022. PMID: 36186170 Free PMC article. - Systemic therapies for malignant pheochromocytoma and paraganglioma can exacerbate hypertension.
Fishbein L, Cohen DL. Fishbein L, et al. J Clin Hypertens (Greenwich). 2013 Jul;15(7):513-4. doi: 10.1111/jch.12106. Epub 2013 Apr 11. J Clin Hypertens (Greenwich). 2013. PMID: 23815541 Free PMC article. No abstract available. - Current Management of Pheochromocytoma/Paraganglioma: A Guide for the Practicing Clinician in the Era of Precision Medicine.
Nölting S, Ullrich M, Pietzsch J, Ziegler CG, Eisenhofer G, Grossman A, Pacak K. Nölting S, et al. Cancers (Basel). 2019 Oct 8;11(10):1505. doi: 10.3390/cancers11101505. Cancers (Basel). 2019. PMID: 31597347 Free PMC article. Review. - Pheochromocytoma/paraganglioma: recent updates in genetics, biochemistry, immunohistochemistry, metabolomics, imaging and therapeutic options.
Antonio K, Valdez MMN, Mercado-Asis L, Taïeb D, Pacak K. Antonio K, et al. Gland Surg. 2020 Feb;9(1):105-123. doi: 10.21037/gs.2019.10.25. Gland Surg. 2020. PMID: 32206603 Free PMC article. Review. - New Perspectives on Pheochromocytoma and Paraganglioma: Toward a Molecular Classification.
Crona J, Taïeb D, Pacak K. Crona J, et al. Endocr Rev. 2017 Dec 1;38(6):489-515. doi: 10.1210/er.2017-00062. Endocr Rev. 2017. PMID: 28938417 Free PMC article. Review.
References
- Beard CM, Sheps SG, Kurland LT, Carney JA, Lie JT. 1983. Occurence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc 58:802–804 - PubMed
- Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, Busaidy N, Cote GJ, Perrier N, Phan A, Patel S, Waguespack S, Jimenez C. 2011. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab 96:717–725 - PubMed
- DeLellis RA, Lloyd RV, Heitz PU, Eng C. 2004. Pathology and genetics: tumours of endocrine organs (IARC WHO Classification of Tumours). Lyon, France: IARC Press
- Plouin PF, Fitzgerald P, Rich T, Ayala-Ramirez M, Perrier ND, Baudin E, Jimenez C. 2012. Metastatic pheochromocytoma and paraganglioma: focus on therapeutics. Horm Metab Res 44:390–399 - PubMed
- Kamio T, Shigematsu K, Sou H, Kawai K, Tsuchiyama H. 1990. Immunohistochemical expression of epidermal growth factor receptors in human adrenocortical carcinoma. Hum Pathol 21:277–282 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical