Substrate-bound crystal structures reveal features unique to Mycobacterium tuberculosis N-acetyl-glucosamine 1-phosphate uridyltransferase and a catalytic mechanism for acetyl transfer - PubMed (original) (raw)
Substrate-bound crystal structures reveal features unique to Mycobacterium tuberculosis N-acetyl-glucosamine 1-phosphate uridyltransferase and a catalytic mechanism for acetyl transfer
Pravin Kumar Ankush Jagtap et al. J Biol Chem. 2012.
Abstract
N-acetyl-glucosamine-1-phosphate uridyltransferase (GlmU), a bifunctional enzyme involved in bacterial cell wall synthesis is exclusive to prokaryotes. GlmU, now recognized as a promising target to develop new antibacterial drugs, catalyzes two key reactions: acetyl transfer and uridyl transfer at two independent domains. Hitherto, we identified GlmU from Mycobacterium tuberculosis (GlmU(Mtb)) to be unique in possessing a 30-residue extension at the C terminus. Here, we present the crystal structures of GlmU(Mtb) in complex with substrates/products bound at the acetyltransferase active site. Analysis of these and mutational data, allow us to infer a catalytic mechanism operative in GlmU(Mtb). In this S(N)2 reaction, His-374 and Asn-397 act as catalytic residues by enhancing the nucleophilicity of the attacking amino group of glucosamine 1-phosphate. Ser-416 and Trp-460 provide important interactions for substrate binding. A short helix at the C-terminal extension uniquely found in mycobacterial GlmU provides the highly conserved Trp-460 for substrate binding. Importantly, the structures reveal an uncommon mode of acetyl-CoA binding in GlmU(Mtb); we term this the U conformation, which is distinct from the L conformation seen in the available non-mycobacterial GlmU structures. Residues, likely determining U/L conformation, were identified, and their importance was evaluated. In addition, we identified that the primary site for PknB-mediated phosphorylation is Thr-418, near the acetyltransferase active site. Down-regulation of acetyltransferase activity upon Thr-418 phosphorylation is rationalized by the structures presented here. Overall, this work provides an insight into substrate recognition, catalytic mechanism for acetyl transfer, and features unique to GlmU(Mtb), which may be exploited for the development of inhibitors specific to GlmU.
Figures
FIGURE 1.
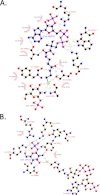
Acetyl-CoA binding to GlmUMtb. A, electron density for the ligands in the structures of GlmUMtb(AcCoA) and GlmUMtb(CoA:GlcN-1-P). In the 2_Fo_ − Fc maps, contoured at 2σ, electron density corresponding to acetyl-CoA (left) and CoA (right) are shown. B, interactions made by acetyl-CoA with residues contributed by the three monomers of the trimer in the structure GlmUMtb(AcCoA) are shown. Letters in parentheses represent the chain identifier of the protein molecule in the trimer. All interactions within 4 Å from acetyl-CoA are shown. Interactions were plotted with LIGPLOT. C, structural superposition of GlmUMtb apo (green) and GlmUMtb(AcCoA) (cyan) structures is shown. Interaction of Tyr-398 with the backbone amino group of acetyl-CoA leads to the ordering of the loop in GlmUMtb(AcCoA) structure.
FIGURE 2.
Interactions between CoA, GlcN-1-P, and the three monomers of the trimer in the structure of GlmUMtb(CoA:GlcN-1-P). A, all of the interactions for CoA are similar to that made by acetyl-CoA in GlmUMtb(AcCoA) structure, except the thiol group of CoA, which forms four new interactions with the hydroxyl group of Ser-416, backbone oxygen of Ala-391, and O1B and N2B groups of GlcN-1-P. B, the phosphate group of GlcN-1-P is stabilized by Lys-403, Arg-344, Lys-362, Tyr-377, and Asn-397, whereas the oxygen and amine groups are stabilized by polar interactions from the protein side chains.
FIGURE 3.
Residues participating in the acetyl transfer reaction. A, the acetyltransferase active site of GlmUMtb reveals probable catalytic residues His-374, Asn-397, and Ser-416, which are highly conserved. Backbone amide of Ala-391 that interacts with the acetyl group is not shown. B, active site mutants were generated, and their acetyltransferase activities were assayed. The activities of H374A and N397A are almost abolished. However, the activity of S416A is not affected significantly. Uridyltransferase activities of the proteins were used as a control. C, a schematic of the proposed acetyltransferase reaction mechanism in GlmUMtb (see text). The amino group activated by His-374 and Asn-397 launches a nucleophilic attack on the carbonyl carbon of acetyl-CoA. Simultaneously, the resultant negative charge on the carbonyl oxygen (oxyanion) is stabilized by the amide backbone of Ala-391.
FIGURE 4.
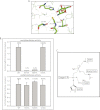
Different conformations of acetyl-CoA bound to GlmUEc and GlmUMtb. A, GlmUEc displays an L confirmation of acetyl-CoA with the adenine base laying flat. A surface representation (see below) shows the adenine base, and backbone phosphates of acetyl-CoA bound to GlmUEc are exposed to the solvent, and the backbone phosphates interacts with the water molecules. B, acetyl-CoA bound to GlmUMtb displays a U conformation with a bent adenine base, whose amine groups interact with the oxygens of the backbone. A surface representation (see below) is shown with the three monomers colored green, red, and yellow. The adenine base of acetyl-CoA bound to GlmUMtb is buried at the interface of two monomers of the trimer. The backbone phosphates interact with Trp-460 and Lys-454 contributed by the tail of the third monomer of the trimer (see Fig. 6_C_). C, superposition of the U and L conformations of acetyl-CoA. It shows a ∼90° bend at the adenine base on the backbone carbon chain in the U conformation. D, comparing the two conformations, Arg-440 in GlmUEc interacts with the backbone oxygens of acetyl-CoA, which prevents the adenine base from bending onto itself. On the other hand, Ala-451 at an analogous position in GlmUMtb is less bulky and hence would not hinder the base from bending. E, the U conformation of acetyl-CoA in GlmUMtb is further stabilized by Arg-439, which provides cation-π interaction to the adenine base. No such stabilizing interaction is seen in GlmUEc, as Thr-428Ec substitutes Arg-439Mtb here. F, comparing the structures of GlmUMtb and GlmUEc two other substitutions viz. Ile-457 to Lys and Arg-455 to Thr were also noted. Together, all of these were believed to reverse the U conformation to an L conformation. G, acetyltransferase activities for mutants R439T, A451R, double mutant (R439T, A451R) and a tetra mutant (A451R,R439T,I457K,R455T) are shown. Uridyltransferase assays were used as internal controls.
FIGURE 5.
Tail truncation experiments identify residues important for acetyltransferase activity. A, acetyltransferase assays show that a complete loss in activity is seen only for Δ37, whereas Δ30 shows an activity comparable with the wild type protein. The right panel depicts the unchanged uridyltransferase activities for the mutants. B, structure analysis of GlmUMtb(AcCoA) structure revealed that Trp-460 and Lys-464 lie in this region and provide important interactions with the backbone phosphate of acetyl-CoA. C, W460A mutant displays complete loss in acetyltransferase activity, whereas K464A does not. The loss in activity of the double mutant (W460A + K464A) reinforces the importance of Trp-460. The control uridyltransferase assays are shown on the right.
FIGURE 6.
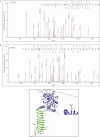
Identification of PknB-mediated phosphorylation site. LC-MS/MS data showing collision-induced fragmentation mass spectra identifying two phosphopeptides in GlmUMtb. pT indicates the site of phosphorylation. An asterisk indicates methionine oxidation. A, MS/MS spectrum of precursor m/z 906.76 (+3) and MH+: 2718.29111 Da, of semitryptic phosphopeptide ILR(pT)QDHEVMAIVEQTDATPSQR. Unambiguous location of the intact phosphate group on Thr-156 was evident by the observation of the “b” ion series containing b6, b10, b11, b12, b13, b14, b15, b17, b18, and b19. B, MS/MS spectrum of precursor m/z 880.40 (+3) and MH+: 2639.20804 Da of phosphopeptide TGSD(pT)MFVAPVTIGDGAYTGAGTVVR. Unambiguous location of the intact phosphate group on Thr-418 was apparent by the observation of the “y” series ion y22 and “b” ion series containing b7, b8, b9, b10, b11, b13, b14, b17, and b19. C, the location of the two threonines Thr-156 and Thr-418 that are phosphorylated are shown (circled) here. Thr-156 is solvent exposed and far from the active sites. Thr-418, the primary site, is closer to the acetyltransferase active site.
FIGURE 7.
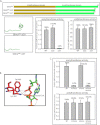
The effect of phosphorylation of GlmUMtb by PknB. A, _in vitro_-phosphorylated GlmUMtb or mutants were digested with trypsin and the resulting phosphopeptides were mapped by two-dimensional resolution on thin layer chromatography. B, Thr-418 was mutated to glutamate (phospho-Thr mimic) and to alanine (phosphorylation negative). Acetyltransferase activities of GlmUMtb-WT and the phospohomutants are shown in the left panel. Corresponding uridyltransferase activities are shown in the right panel. C, the acetyltransferase domain, which consists of the β-helix, has a highly hydrophobic core (gray residues) and a few polar residues. Thr-418 is shown in yellow. Hydroxyl group of Thr-418 makes a hydrogen bond with the backbone oxygen of Gly-415. Mutation of Thr-418 to alanine (T418A) would disrupt this interaction (black arrow), whereas mutation to a glutamate (T418E) would introduce steric clashes in the region as shown by red patches. However, mutation to serine (T418S) would preserve the polar interaction with the backbone of Gly-415. The hydroxyl and backbone amine group of Ser-416 interacts with the backbone oxygen of the acetyl-CoA. It is expected that perturbing these interactions would affect the positioning of the acetyl group for catalysis.
Similar articles
- PknB-mediated phosphorylation of a novel substrate, N-acetylglucosamine-1-phosphate uridyltransferase, modulates its acetyltransferase activity.
Parikh A, Verma SK, Khan S, Prakash B, Nandicoori VK. Parikh A, et al. J Mol Biol. 2009 Feb 20;386(2):451-64. doi: 10.1016/j.jmb.2008.12.031. Epub 2008 Dec 24. J Mol Biol. 2009. PMID: 19121323 - Expression, essentiality, and a microtiter plate assay for mycobacterial GlmU, the bifunctional glucosamine-1-phosphate acetyltransferase and N-acetylglucosamine-1-phosphate uridyltransferase.
Zhang W, Jones VC, Scherman MS, Mahapatra S, Crick D, Bhamidi S, Xin Y, McNeil MR, Ma Y. Zhang W, et al. Int J Biochem Cell Biol. 2008;40(11):2560-71. doi: 10.1016/j.biocel.2008.05.003. Epub 2008 May 15. Int J Biochem Cell Biol. 2008. PMID: 18573680 Free PMC article. - Unique C-terminal extension and interactome of Mycobacterium tuberculosis GlmU impacts it's in vivo function and the survival of pathogen.
Agarwal M, Soni V, Kumar S, Singha B, Nandicoori VK. Agarwal M, et al. Biochem J. 2021 Jun 11;478(11):2081-2099. doi: 10.1042/BCJ20210170. Biochem J. 2021. PMID: 33955473 - GlmU Inhibitors as Promising Antibacterial Agents: A Review.
Palathoti N, Azam MA. Palathoti N, et al. Mini Rev Med Chem. 2023;23(3):343-360. doi: 10.2174/1389557522666220817114445. Mini Rev Med Chem. 2023. PMID: 35980047 Review. - Mechanism and Potential Inhibitors of GlmU: A Novel Target for Antimicrobial Drug Discovery.
Sharma R, Khan IA. Sharma R, et al. Curr Drug Targets. 2017;18(14):1587-1597. doi: 10.2174/1389450117666160502152011. Curr Drug Targets. 2017. PMID: 27138757 Review.
Cited by
- Archaeal pseudomurein and bacterial murein cell wall biosynthesis share a common evolutionary ancestry.
Subedi BP, Martin WF, Carbone V, Duin EC, Cronin B, Sauter J, Schofield LR, Sutherland-Smith AJ, Ronimus RS. Subedi BP, et al. FEMS Microbes. 2021 Aug 24;2:xtab012. doi: 10.1093/femsmc/xtab012. eCollection 2021. FEMS Microbes. 2021. PMID: 37334239 Free PMC article. - Depletion of M. tuberculosis GlmU from Infected Murine Lungs Effects the Clearance of the Pathogen.
Soni V, Upadhayay S, Suryadevara P, Samla G, Singh A, Yogeeswari P, Sriram D, Nandicoori VK. Soni V, et al. PLoS Pathog. 2015 Oct 21;11(10):e1005235. doi: 10.1371/journal.ppat.1005235. eCollection 2015 Oct. PLoS Pathog. 2015. PMID: 26489015 Free PMC article. - The Mechanism of Acetyl Transfer Catalyzed by Mycobacterium tuberculosis GlmU.
Craggs PD, Mouilleron S, Rejzek M, de Chiara C, Young RJ, Field RA, Argyrou A, de Carvalho LPS. Craggs PD, et al. Biochemistry. 2018 Jun 19;57(24):3387-3401. doi: 10.1021/acs.biochem.8b00121. Epub 2018 May 2. Biochemistry. 2018. PMID: 29684272 Free PMC article. - The Mycobacterial Cell Wall--Peptidoglycan and Arabinogalactan.
Alderwick LJ, Harrison J, Lloyd GS, Birch HL. Alderwick LJ, et al. Cold Spring Harb Perspect Med. 2015 Mar 27;5(8):a021113. doi: 10.1101/cshperspect.a021113. Cold Spring Harb Perspect Med. 2015. PMID: 25818664 Free PMC article. Review. - Targeting the Heart of Mycobacterium: Advances in Anti-Tubercular Agents Disrupting Cell Wall Biosynthesis.
Diab A, Dickerson H, Al Musaimi O. Diab A, et al. Pharmaceuticals (Basel). 2025 Jan 9;18(1):70. doi: 10.3390/ph18010070. Pharmaceuticals (Basel). 2025. PMID: 39861133 Free PMC article. Review.
References
- Anderson M. S., Raetz C. R. (1987) Biosynthesis of lipid A precursors in Escherichia coli. A cytoplasmic acyltransferase that converts UDP-N-acetylglucosamine to UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine. J. Biol. Chem. 262, 5159–5169 - PubMed
- Mengin-Lecreulx D., van Heijenoort J. (1994) Copurification of glucosamine-1-phosphate acetyltransferase and N-acetylglucosamine-1-phosphate uridyltransferase activities of Escherichia coli: characterization of the glmU gene product as a bifunctional enzyme catalyzing two subsequent steps in the pathway for UDP-N-acetylglucosamine synthesis. J. Bacteriol. 176, 5788–5795 - PMC - PubMed
- Sassetti C. M., Boyd D. H., Rubin E. J. (2003) Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48, 77–84 - PubMed
- Zhang W., Jones V. C., Scherman M. S., Mahapatra S., Crick D., Bhamidi S., Xin Y., McNeil M. R., Ma Y. (2008) Expression, essentiality, and a microtiter plate assay for mycobacterial GlmU, the bifunctional glucosamine-1-phosphate acetyltransferase and N-acetylglucosamine-1-phosphate uridyltransferase. Int. J. Biochem. Cell Biol. 40, 2560–2571 - PMC - PubMed
- Olsen L. R., Roderick S. L. (2001) Structure of the Escherichia coli GlmU pyrophosphorylase and acetyltransferase active sites. Biochemistry 40, 1913–1921 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources