B7x in the periphery abrogates pancreas-specific damage mediated by self-reactive CD8 T cells - PubMed (original) (raw)
. 2012 Oct 15;189(8):4165-74.
doi: 10.4049/jimmunol.1201241. Epub 2012 Sep 12.
Lisa Scandiuzzi, Anjana Ray, Joyce Wei, Kimberly A Hofmeyer, Yael M Abadi, P'ng Loke, Juan Lin, Jianda Yuan, David V Serreze, James P Allison, Xingxing Zang
Affiliations
- PMID: 22972920
- PMCID: PMC3466330
- DOI: 10.4049/jimmunol.1201241
B7x in the periphery abrogates pancreas-specific damage mediated by self-reactive CD8 T cells
Jun Sik Lee et al. J Immunol. 2012.
Abstract
B7x (B7-H4 or B7S1) is the seventh member of the B7 family, and its in vivo function remains largely unknown. Despite new genetic data linking the B7x gene with autoimmune diseases, how exactly it contributes to peripheral tolerance and autoimmunity is unclear. In this study, we showed that B7x protein was not detected on APCs or T cells in both human and mice, which is unique in the B7 family. Because B7x protein is expressed in some peripheral cells such as pancreatic β cells, we used a CD8 T cell-mediated diabetes model (AI4αβ) in which CD8 T cells recognize an endogenous self-Ag, and found that mice lacking B7x developed more severe diabetes than control AI4αβ mice. Conversely, mice overexpressing B7x in the β cells (Rip-B7xAI4αβ) were diabetes free. Furthermore, adoptive transfer of effector AI4αβ CD8 T cells induced diabetes in control mice, but not in Rip-B7xAI4αβ mice. Mechanistic studies revealed that pathogenic effector CD8 T cells were capable of migrating to the pancreas but failed to robustly destroy tissue when encountering local B7x in Rip-B7xAI4αβ mice. Although AI4αβ CD8 T cells in Rip-B7xAI4αβ and AI4αβ mice showed similar cytotoxic function, cell death, and global gene expression profiles, these cells had greater proliferation in AI4αβ mice than in RIP-B7xAI4αβ mice. These results suggest that B7x in nonlymphoid organs prevents peripheral autoimmunity partially through inhibiting proliferation of tissue-specific CD8 T cells, and that local overexpression of B7x on pancreatic β cells is sufficient to abolish CD8 T cell-induced diabetes.
Figures
FIGURE 1
B7x deficiency leads to exacerbated diabetes whereas B7x overexpression in pancreatic β cells abrogates disease induced by CD8 T cells recognizing an endogenous self-antigen in the pancreas. (A) Scheme for generation of B7x−/−AI4αβ, Rip-B7xAI4αβ and AI4αβ mice on C57BL/6.H2g7/g7 background. (B–D) B7x−/−AI4αβ, Rip-B7xAI4αβ, and control AI4αβ mice were examined for diabetes incidence (B), glucose measurements (C), and survival (D); n = 8–10 mice per group. Data are representative of three independent experiments and are presented as means ± S.E.M.
FIGURE 2
Pathogenic CD8 T cells infiltrate into the pancreas in B7x-transgenic mice. (A) HE and GAF stained pancreatic sections from C57BL/6.H2g7/g7, AI4αβ, and RIP-B7xAI4αβ mice. Scale bar 50 µm, magnification 20×. (B) HE stained sections of brain, kidney, liver, and lung fromC57BL/6.H2g7/g7, AI4αβ, and RIP-B7xAI4αβ mice. Scale bar 200 µm, magnification 5×. n = 3–5 mice per group, data represent one of three independent experiments.
FIGURE 3
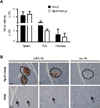
Leukocytic infiltrates and B7 expression in islets of B7x-transgenic mice. (A) Absolute number of AI4αβ CD8 T cells normalized to weight of spleen, pancreatic lymph node (PLN), and pancreas from AI4αβ and RIP-B7xAI4αβ mice. AI4αβ CD8 T cells were determined by MimA2/H2-Db tetramer and anti-CD8 staining. n = 3–5 mice per group; data are presented as means ± SEM and they represent one of three independent experiments. (B) Representative pictures of immunohistochemistry staining with anti-B7x Ab and isotype control in pancreatic tissue sections of AI4αβ and RIP-B7xAI4αβ mice. Some islets of Rip-B7xAI4αβ mice had leukocytic infiltrates where B7x-positive cells were confined to areas devoid of infiltrates. Islets in AI4αβ mice were barely detectable with no obvious staining for B7x. Hematoxylin staining of nuclei in images is blue, Ab-specific staining of B7x is brown. Scale bar 50µm, magrification 20×. Circles and arrows indicate islets.
FIGURE 4
Irradiated Rip-B7x mice do not develop diabetes after adoptive transfer of effector AI4αβ CD8 T cells. (A) Scheme of adoptive transferred AI4αβ splenocytes-induced diabetes in irradiated recipients. (B–C) Irradiated Rip-B7xB6.H2g7/g7 mice and control C57BL/6.H2g7/g7 mice were intravenously injected with splenocytes from AI4αβ mice and monitored for diabetes development including diabetes incidence (B) and urine glucose level (C). (D) Representative pictures of HE and GAF stained pancreatic tissue sections 30 days after adopted transfer of AI4αβ cells in Rip-B7xB6.H2g7/g7 mice and C57BL/6.H2g7/g7 mice. Scale bars, 50 µm, magnification 20× (left panels); 100 µm, magnification 10x (right panels). Data are presented as means ± SEM and they represent one of four independent experiments; n=10 per group.
FIGURE 5
B7x inhibits effector CD8 T cell-induced diabetes. (A) Scheme for adoptive transfer of effector CD8 T cell-induced diabetes in naïve mice. (B–C) Naive Rip-B7xB6.H2g7/g7 mice and control C57BL/6.H2g7/g7 mice were intravenously injected with effector AI4αβ CD8 T cells and monitored for diabetes development including diabetes incidence (B) and urine glucose level (C). (D) Absolute number of AI4αβ cells in spleen, pancreatic lymph node (PLN), and pancreas from C57BL/6.H2g7/g7 mice and Rip-B7xB6.H2g7/g7 mice 30 days after adoptive transfer of effector CD8 T cells. Data are shown as mean ± SEM (n=10 per group) and they represent one of three independent experiments. * p <0.05 by Student t test.
FIGURE 6
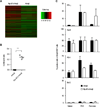
Comparison of global gene expression profiles and effector functional proteins in pancreatic CD8 T cells. (A) Hierarchical clustering analysis for top 500 genes with the lowest adjusted p value in pancreatic AI4αβ CD8 T cells from Rip-B7xAI4αβ and AI4αβ mice, three replicates per group are shown. (B) RT-PCR determination of mRNAs for CCL3 and the housekeeping gene β-actin in pancreatic AI4αβ cells isolated from AI4αβ and Rip-B7xAI4αβ mice at 4–5 weeks of age. (C) Flow cytometry analysis of cell surface PD-1 and intracellular IFN-γ, GrzB, and Bcl-2 expressed by AI4αβ cells in spleen, pancreatic lymph node (PLN), and pancreas from RIP-B7xAI4αβ and AI4αβ mice. AI4αβ CD8 T cells were determined by MimA2/H2-Db tetramer and anti-CD8 staining. Data are shown as mean ± SEM (n=3–4 per group) of two independent experiments. ***p <0.001.
FIGURE 7
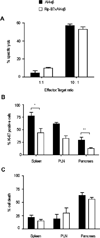
Cytotoxic function, proliferation, and cell death of AI4αβ CD8 T cells. (A) AI4αβ CD8 T cell cytotoxic response toward target cells of MimA2 peptide-pulsed C57BL/6.H2g7/g7 splenocytes. (B) Percentage of AI4αβ T cells that expressed Ki-67 in spleen, PLN, and pancreas from AI4αβ and RIP-B7xAI4αβ mice. AI4αβ CD8 T cells were determined by MimA2/H2-Db tetramer and anti-CD8 staining. (C) Percentage of cell death in AI4αβ CD8 T cells. Data are shown as means ± SEM and they are representative of two to three independent experiments; n = 3–5 mice per group. *p <0.05, **p <0.01 by Student t test.
Similar articles
- B7x-from bench to bedside.
Kaur G, Janakiram M. Kaur G, et al. ESMO Open. 2019 Sep 8;4(5):e000554. doi: 10.1136/esmoopen-2019-000554. eCollection 2019. ESMO Open. 2019. PMID: 31555486 Free PMC article. Review. - Tissue-specific expression of B7x protects from CD4 T cell-mediated autoimmunity.
Wei J, Loke P, Zang X, Allison JP. Wei J, et al. J Exp Med. 2011 Aug 1;208(8):1683-94. doi: 10.1084/jem.20100639. Epub 2011 Jul 4. J Exp Med. 2011. PMID: 21727190 Free PMC article. - Tissue-expressed B7x affects the immune response to and outcome of lethal pulmonary infection.
Hofmeyer KA, Scandiuzzi L, Ghosh K, Pirofski LA, Zang X. Hofmeyer KA, et al. J Immunol. 2012 Sep 15;189(6):3054-63. doi: 10.4049/jimmunol.1200701. Epub 2012 Aug 1. J Immunol. 2012. PMID: 22855708 Free PMC article. - B7x/B7-H4 modulates the adaptive immune response and ameliorates renal injury in antibody-mediated nephritis.
Pawar RD, Goilav B, Xia Y, Herlitz L, Doerner J, Chalmers S, Ghosh K, Zang X, Putterman C. Pawar RD, et al. Clin Exp Immunol. 2015 Feb;179(2):329-43. doi: 10.1111/cei.12452. Clin Exp Immunol. 2015. PMID: 25205493 Free PMC article. - The B7x Immune Checkpoint Pathway: From Discovery to Clinical Trial.
John P, Wei Y, Liu W, Du M, Guan F, Zang X. John P, et al. Trends Pharmacol Sci. 2019 Nov;40(11):883-896. doi: 10.1016/j.tips.2019.09.008. Epub 2019 Oct 31. Trends Pharmacol Sci. 2019. PMID: 31677920 Free PMC article. Review.
Cited by
- Review and Meta-Analyses of TAAR1 Expression in the Immune System and Cancers.
Fleischer LM, Somaiya RD, Miller GM. Fleischer LM, et al. Front Pharmacol. 2018 Jun 26;9:683. doi: 10.3389/fphar.2018.00683. eCollection 2018. Front Pharmacol. 2018. PMID: 29997511 Free PMC article. - Nardilysin-dependent proteolysis of cell-associated VTCN1 (B7-H4) marks type 1 diabetes development.
Radichev IA, Maneva-Radicheva LV, Amatya C, Parker C, Ellefson J, Wasserfall C, Atkinson M, Burn P, Savinov AY. Radichev IA, et al. Diabetes. 2014 Oct;63(10):3470-82. doi: 10.2337/db14-0213. Epub 2014 May 21. Diabetes. 2014. PMID: 24848066 Free PMC article. - B7-H4 Polymorphism Influences the Prevalence of Diabetes Mellitus and Pro-Atherogenic Dyslipidemia in Patients with Psoriasis.
Yang W, Huang Q, Han L, Wang B, Yawalkar N, Zhang Z, Yan K. Yang W, et al. J Clin Med. 2022 Oct 22;11(21):6235. doi: 10.3390/jcm11216235. J Clin Med. 2022. PMID: 36362461 Free PMC article. - B7x-from bench to bedside.
Kaur G, Janakiram M. Kaur G, et al. ESMO Open. 2019 Sep 8;4(5):e000554. doi: 10.1136/esmoopen-2019-000554. eCollection 2019. ESMO Open. 2019. PMID: 31555486 Free PMC article. Review. - Structure and cancer immunotherapy of the B7 family member B7x.
Jeon H, Vigdorovich V, Garrett-Thomson SC, Janakiram M, Ramagopal UA, Abadi YM, Lee JS, Scandiuzzi L, Ohaegbulam KC, Chinai JM, Zhao R, Yao Y, Mao Y, Sparano JA, Almo SC, Zang X. Jeon H, et al. Cell Rep. 2014 Nov 6;9(3):1089-98. doi: 10.1016/j.celrep.2014.09.053. Epub 2014 Oct 30. Cell Rep. 2014. PMID: 25437562 Free PMC article.
References
- Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–873. - PubMed
- Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. - PubMed
- Rai E, Wakeland EK. Genetic predisposition to autoimmunity--what have we learned? Semin Immunol. 2011;23:67–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DK007513/DK/NIDDK NIH HHS/United States
- P60DK020541/DK/NIDDK NIH HHS/United States
- R01 DK046266/DK/NIDDK NIH HHS/United States
- DP2 DK083076/DK/NIDDK NIH HHS/United States
- R01 DK095735/DK/NIDDK NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- P30CA013330/CA/NCI NIH HHS/United States
- T32DK007513/DK/NIDDK NIH HHS/United States
- P60 DK020541/DK/NIDDK NIH HHS/United States
- DP2DK083076/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous