Rab33a mediates anterograde vesicular transport for membrane exocytosis and axon outgrowth - PubMed (original) (raw)
Rab33a mediates anterograde vesicular transport for membrane exocytosis and axon outgrowth
Hitomi Nakazawa et al. J Neurosci. 2012.
Abstract
Axon outgrowth requires plasma membrane expansion, which results from post-Golgi vesicular transport and fusion. However, the molecular mechanisms regulating post-Golgi vesicular trafficking for membrane expansion and axon outgrowth remain unclear. Here, we show that Rab33a expression became upregulated during axon outgrowth of cultured rat hippocampal neurons. Rab33a was preferentially localized to the Golgi apparatus and to synaptophysin-positive vesicles that are transported along the growing axon. Previous studies showed that synaptophysin is localized to post-Golgi vesicles transported by fast axonal transport in developing neurons. Reduction of Rab33a expression by RNAi (RNA interference) inhibited the anterograde transport of synaptophysin-positive vesicles, leading to their decrease in axonal tips. Furthermore, this treatment reduced membrane fusion of synaptophysin-positive vesicles at the growth cones and inhibited axon outgrowth. Overexpression of Rab33a, on the other hand, induced excessive accumulation of synaptophysin-positive vesicles and concurrent formation of surplus axons. These data suggest that Rab33a participates in axon outgrowth by mediating anterograde axonal transport of synaptophysin-positive vesicles and their concomitant fusion at the growth cones.
Figures
Figure 1.
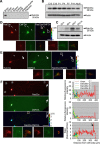
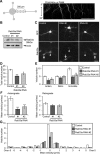
Expression and intracellular localization of Rab33a. A, Immunoblot analysis of Rab33a in adult rat tissues. B, C, Immunoblot analysis of Rab33a in developing rat brain (B) and in rat cultured hippocampal neurons at stage 2 (cultured for 22 h), stage 3 (cultured for 65 h), stage 4 (cultured for 7 d), and stage 5 (cultured for 14 d) (C). Immunoblots with anti-actin antibody served as loading controls. D–F, Immunofluorescence localization of Rab33a in stage 2 (D), stage 3 (E), and stage 4 (F) hippocampal neurons. Neurons were double-stained by anti-Rab33a antibody (red) and the volume marker CMFDA (green). Pseudo-color images show the relative concentration of Rab33a (Rab33a immunoreactivity/CMFDA staining). Quantitative profiles show the relative fluorescence intensities of Rab33a (top) and CMFDA (middle) and the relative concentration of Rab33a (bottom). Arrows and arrowheads denote axonal growth cones and minor process growth cones, respectively. Insets in D–F show enlarged views of the growth cone in the rectangles. Scale bars: D, E, 20 μm; F, 50 μm.
Figure 2.
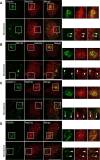
Rab33a is localized to the Golgi apparatus. A–D, Immunofluorescence localization of Rab33a and the Golgi markers giantin (A), GM130 (B), adaptin γ (C), and TGN38 (D) in the cell bodies of hippocampal neurons under control (top) or nocodazole (bottom) treatment. Arrowheads indicate colocalization of Rab33a with the Golgi markers. Scale bar, 10 μm.
Figure 3.
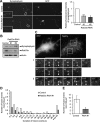
Rab33a is localized to synaptophysin-positive vesicles transported along axons. A, A stage 3 hippocampal neuron expressing EGFP-Rab33a was observed under a time-lapse fluorescence microscope every 1 s (left). The kymographs (right) of the three neurites show anterograde and retrograde transport of EGFP-Rab33a-positive vesicles. Images were captured every 1 s for 150 s. B, E, Immunofluorescence localization of Rab33a (red) and synaptophysin (green) in an axon shaft (B) and a growth cone (E) of a cultured hippocampal neuron. Arrowheads denote colocalization of Rab33a and synaptophysin. C, Cotransport of EGFP-Rab33a with RFP-synaptophysin in an axon of a hippocampal neuron observed by confocal microscopy. A vesicle bearing both EGFP-Rab33a and RFP-synaptophysin is indicated by arrows. D, Copurification of Rab33a with synaptophysin-containing vesicles of cultured hippocampal neurons. Immunoaffinity purification of synaptophysin-containing vesicles was performed, and the purified fractions were analyzed by immunoblot with anti-Rab33a antibody. Scale bars: A, 10 μm; B, C, E, 5 μm.
Figure 4.
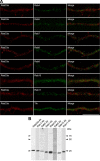
Subcellular localization of Rab33a in axons of cultured hippocampal neurons. A, Immunofluorescence localization of Rab33a (red) and early endosome markers Rab4, Rab5, and Rab10, late endosome markers Rab7 and Rab9, recycling endosome markers Rab8 and Rab11, and Trk (green). Note the distinct localizations of Rab33a and these proteins. B, Immunoblot analyses to test the specificity of the antibodies used in A. All antibodies recognized major bands at the correct molecular weights of the target molecules, thereby suggesting that the antibodies recognize their antigens. The anti-Rab5, Rab8, and Rab9 antibodies recognized faint bands at ∼75, 90, and 50 kDa, respectively, in addition to the main band, indicating that they interact with additional unknown molecules. However, since the data in A show that the immunoreactivity of anti-Rab5, Rab8, and Rab9 antibodies does not overlap with that of anti-Rab33a antibody, Rab33a is unlikely to colocalize with Rab5, Rab8, Rab9, or the unknown molecules in the axons. Scale bar, 10 μm.
Figure 5.
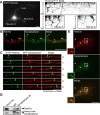
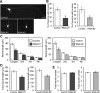
Rab33a is involved in the anterograde axonal transport of synaptophysin-positive vesicles. A, A schematic illustration of the region of observation of mRFP-synaptophysin-positive vesicles (left) and a kymograph of the transport (right). Images were captured every 100 ms for 20 s in an axon. B, Suppression of Rab33a by miRNAi in hippocampal neurons. Proteins extracted from hippocampal neurons expressing control miRNA or miRNA against Rab33a (#1 or #2) were subjected to SDS-PAGE and the amount of Rab33a in each sample was examined by immunoblot. C, Rab33a immunoreactivity of neurons transfected with control miRNA or miRNA against Rab33a (#1 or #2). Neurons were fixed at 36 h in culture. The immunoreactivity of Rab33a in GFP-positive neurons was compared with that of control miRNA neurons. The arrowheads and arrows indicate GFP-positive transfected and untransfected neurons, respectively. D, Total vesicle number observed in the boxed region in A. Error bars indicate SEM. *p < 0.05 and ***p < 0.005 relative to control, Student's t tests. E, The number of anterograde, retrograde, and immobile vesicles observed in the region in A. Error bars indicate SEM. *p < 0.05 and **p < 0.01 relative to control, Student's t tests. F, Mean velocities of anterograde (left) and retrograde (right) transport of mRFP-synaptophysin-positive vesicles. Error bars indicate SEM. ***p < 0.005 relative to control, Student's t tests. G, The number of vesicles with indicated velocities observed in the region in A. A total of 450 vesicles in 23 neurons for control miRNA, 318 vesicles in 27 neurons for Rab33a miRNA #1, and 321 vesicles in 22 neurons for Rab33a miRNA #2 were examined in four independent experiments. Scale bars: A, 50 μm; C, 20 μm.
Figure 6.
Rab33a is involved in fusion of synaptophysin-positive vesicles at the growth cone. A, Synaptophysin immunoreactivity of neurons transfected with control miRNA or miRNA against Rab33a. Insets in A show enlarged views of the growth cones in the rectangles. Relative fluorescence intensity of synaptophysin in the distal region of the axon (20 μm length, rectangles) was quantified. A total of 108 neurons for control miRNA, 104 neurons for Rab33a miRNA #1, and 89 neurons for Rab33a miRNA #2 were examined in three independent experiments. Error bars indicate SEM. *p < 0.05 and ***p < 0.005 relative to control, Student's t tests. B, Immunoblot analyses of endogenous synaptophysin and Rab33a in cultured neurons transfected with control miRNA, miRNA against Rab33a #1, or miRNA against Rab33a #2. C–E, Hippocampal neurons were transfected with SypHy and observed using TIRF microscopy. C, A growth cone of a hippocampal neuron expressing RFP and SypHy (top). Representative images from a time-lapse series (bottom) display vesicle fusion events at the indicated areas 1 and 2 of the growth cone (arrowheads). D, Histogram of the duration of SypHy signals in growth cones cotransfected with SypHy and Rab33a miRNA #1 or control miRNA. E, Frequency of vesicle fusion in the growth cones was quantified. A total of 227 vesicles in 12 growth cones for control miRNA and 111 vesicles in 11 growth cones for Rab33a miRNA #1 were examined. Error bars indicate SEM. *p < 0.05 relative to control, Student's t tests. Scale bars: A, 20 μm; C, 10 μm.
Figure 7.
Suppression of Rab33a expression inhibits axon formation and axon outgrowth. A–E, Morphological analyses of Rab33a RNAi-treated neurons. A, Neurons visualized by the fluorescence of GFP. B, Percentage of neurons with an axon. C, Neurite length of Rab33a RNAi-treated neurons. D, Total neurite length. E, Total neurite number. Neurons transfected with control miRNA, Rab33a miRNA #1, or Rab33a miRNA #2 were fixed at 36 h in culture and immunostained by anti-Rab33a and anti-GFP antibodies. In the analyses with miRNA #1, a total of 386 neurons for control and 332 neurons for Rab33a RNAi #1 were examined in six independent experiments. In the analyses with miRNA #2, a total of 276 neurons for control and 238 neurons for Rab33a RNAi #2 were examined in four independent experiments. Error bars indicate SEM. *p < 0.05, **p < 0.01, ***p < 0.005 relative to control, Student's t tests. Scale bar, 20 μm.
Figure 8.
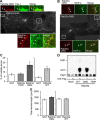
Overexpression of Rab33a induces formation of multiple axons. A, Hippocampal neurons overexpressing Myc-Rab33a Q95L were cultured for 6 d and then immunostained with anti-Myc (red) and tau-1 (green) antibodies. Arrowheads in the enlargements of the two boxed areas of the main image indicate axons labeled by tau-1 antibody. B, Hippocampal neurons overexpressing Myc-Rab33a Q95L were cultured for 6 d and then double immunostained with anti-Myc (red) and anti-MAP-2 (green) antibodies. Arrowheads in the enlarged images indicate axons unlabeled by anti-MAP-2 antibody. C, Percentage of neurons with multiple axons. Neurons transfected with Myc-GST, Myc-Rab33a Q95L, Myc-Rab33a T50R, or Myc-Rab33a WT were fixed on DIV6 and analyzed. A total of 127 neurons for GST, 120 neurons for Rab33a WT (right), 74 neurons for GST, 83 neurons for Rab33a T50R, and 76 neurons for Rab33a Q95L (left) were examined in three independent experiments. Error bars indicate SEM. *p < 0.05 relative to GST or Rab33a T50R, one-way ANOVA analysis with Schaffer's post hoc test (left). *p < 0.05 relative to GST, Student's t tests (right). D, Guanine nucleotide-bound form of WT and mutants of Rab33a expressed in HEK293T cells. HEK293T cells were transfected with expression vectors encoding the N-terminally FLAG-tagged Rab33a proteins indicated at the bottom and metabolically radiolabeled with 32Pi. FLAG-Rab33a proteins were immunoprecipitated with an anti-FLAG M2 monoclonal antibody. Guanine nucleotides bound to Flag-Rab33a proteins were separated by thin-layer chromatography. E, Total neurite length of neurons overexpressing Myc-GST, Myc-Rab33a WT, or Myc-Rab33a Q95L on DIV6. Error bars indicate SEM. Student's t tests. Scale bars: A, B, 50 μm.
Figure 9.
Overexpression of Rab33a induces excessive accumulation of synaptophysin-positive vesicles. A, Hippocampal neurons overexpressing Myc-Rab33a Q95L were cultured for 6 d and then immunostained with anti-Myc (red) and anti-synaptophysin (green) antibodies. Arrowheads in the enlargements of the two boxed areas of the main image indicate axons labeled by anti-synaptophysin antibody. B, Synaptophysin immunoreactivity of short neurites (<100 μm) of cultured hippocampal neurons overexpressing Myc-GST, Myc-Rab33a WT, or Myc-Rab33a Q95L (left). Neurons were fixed on DIV3 and costained with the volume marker CMAC. Relative synaptophysin immunoreactivity in the distal region of minor processes (20 μm length, rectangles) was quantified (right graph). Arrows indicate the axons. A total of 61 neurons for GST, 70 neurons for Rab33a WT, and 66 neurons for Rab33a QL were examined in three independent experiments. Error bars indicate SEM. *p < 0.05 and ***p < 0.005 relative to GST, Student's t tests. C, Immunoblot analysis of endogenous synaptophysin in cultured neurons overexpressing Myc-GST, Myc-Rab33a Q95L, or Myc-Rab33a WT. Scale bars: A, 50 μm; B, 20 μm.
Similar articles
- Rab33a and Rab33ba mediate the outgrowth of forebrain commissural axons in the zebrafish brain.
Huang L, Urasaki A, Inagaki N. Huang L, et al. Sci Rep. 2019 Feb 12;9(1):1799. doi: 10.1038/s41598-018-38468-5. Sci Rep. 2019. PMID: 30755680 Free PMC article. - Cdk5 Regulation of the GRAB-Mediated Rab8-Rab11 Cascade in Axon Outgrowth.
Furusawa K, Asada A, Urrutia P, Gonzalez-Billault C, Fukuda M, Hisanaga SI. Furusawa K, et al. J Neurosci. 2017 Jan 25;37(4):790-806. doi: 10.1523/JNEUROSCI.2197-16.2016. J Neurosci. 2017. PMID: 28123016 Free PMC article. - GTP hydrolysis of TC10 promotes neurite outgrowth through exocytic fusion of Rab11- and L1-containing vesicles by releasing exocyst component Exo70.
Fujita A, Koinuma S, Yasuda S, Nagai H, Kamiguchi H, Wada N, Nakamura T. Fujita A, et al. PLoS One. 2013 Nov 4;8(11):e79689. doi: 10.1371/journal.pone.0079689. eCollection 2013. PLoS One. 2013. PMID: 24223996 Free PMC article. - Vesicular traffic: an integral part of plant life.
Ueda T, Nakano A. Ueda T, et al. Curr Opin Plant Biol. 2002 Dec;5(6):513-7. doi: 10.1016/s1369-5266(02)00299-6. Curr Opin Plant Biol. 2002. PMID: 12393014 Review. - Membrane turnover and receptor trafficking in regenerating axons.
Hausott B, Klimaschewski L. Hausott B, et al. Eur J Neurosci. 2016 Feb;43(3):309-17. doi: 10.1111/ejn.13025. Epub 2015 Aug 19. Eur J Neurosci. 2016. PMID: 26222895 Review.
Cited by
- SCYL1 arginine methylation by PRMT1 is essential for neurite outgrowth via Golgi morphogenesis.
Amano G, Matsuzaki S, Mori Y, Miyoshi K, Han S, Shikada S, Takamura H, Yoshimura T, Katayama T. Amano G, et al. Mol Biol Cell. 2020 Aug 15;31(18):1963-1973. doi: 10.1091/mbc.E20-02-0100. Epub 2020 Jun 17. Mol Biol Cell. 2020. PMID: 32583741 Free PMC article. - Rab33a and Rab33ba mediate the outgrowth of forebrain commissural axons in the zebrafish brain.
Huang L, Urasaki A, Inagaki N. Huang L, et al. Sci Rep. 2019 Feb 12;9(1):1799. doi: 10.1038/s41598-018-38468-5. Sci Rep. 2019. PMID: 30755680 Free PMC article. - Rab43 GTPase directs postsynaptic trafficking and neuron-specific sorting of G protein-coupled receptors.
Wei Z, Xu X, Fang Y, Khater M, Naughton SX, Hu G, Terry AV Jr, Wu G. Wei Z, et al. J Biol Chem. 2021 Jan-Jun;296:100517. doi: 10.1016/j.jbc.2021.100517. Epub 2021 Mar 4. J Biol Chem. 2021. PMID: 33676895 Free PMC article. - Transcriptomic Analysis of Ovaries from Pigs with High And Low Litter Size.
Zhang X, Huang L, Wu T, Feng Y, Ding Y, Ye P, Yin Z. Zhang X, et al. PLoS One. 2015 Oct 1;10(10):e0139514. doi: 10.1371/journal.pone.0139514. eCollection 2015. PLoS One. 2015. PMID: 26426260 Free PMC article. - News about non-secretory exocytosis: mechanisms, properties, and functions.
D'Alessandro R, Meldolesi J. D'Alessandro R, et al. J Mol Cell Biol. 2019 Sep 19;11(9):736-746. doi: 10.1093/jmcb/mjy084. J Mol Cell Biol. 2019. PMID: 30605539 Free PMC article. Review.
References
- Arimura N, Kaibuchi K. Key regulators in neuronal polarity. Neuron. 2005;48:881–884. - PubMed
- Arimura N, Kimura T, Nakamuta S, Taya S, Funahashi Y, Hattori A, Shimada A, Ménager C, Kawabata S, Fujii K, Iwamatsu A, Segal RA, Fukuda M, Kaibuchi K. Anterograde transport of TrkB in axons is mediated by direct interaction with Slp1 and Rab27. Dev Cell. 2009;16:675–686. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials