Accumulation and toxicity of antibody-targeted doxorubicin-loaded PEG-PE micelles in ovarian cancer cell spheroid model - PubMed (original) (raw)
Accumulation and toxicity of antibody-targeted doxorubicin-loaded PEG-PE micelles in ovarian cancer cell spheroid model
Federico Perche et al. J Control Release. 2012.
Abstract
We describe the evaluation of doxorubicin-loaded PEG-PE micelles targeting using an ovarian cancer cell spheroid model. Most ovarian cancer patients present at an advanced clinical stage and develop resistance to standard of care platinum/taxane therapy. Doxorubicin is also approved for ovarian cancer but had limited benefits in refractory patients. In this study, we used drug-resistant spheroid cultures of ovarian carcinoma to evaluate the uptake and cytotoxicity of an antibody-targeted doxorubicin formulation. Doxorubicin was encapsulated in polyethylene glycol-phosphatidyl ethanolamine (PEG-PE) conjugated micelles. The doxorubicin-loaded PEG-PE micelles (MDOX) were further decorated with a cancer cell-specific monoclonal 2C5 antibody to obtain doxorubicin-loaded immunomicelles (2C5-MDOX). Targeting and resulting toxicity of doxorubicin-loaded PEG-PE micelles were evaluated in three dimensional cancer cell spheroids. Superior accumulation of 2C5-MDOX compared to free doxorubicin or untargeted MDOX in spheroids was evidenced both by flow cytometry, fluorescence and confocal microscopy. Interestingly, even higher toxicity was measured by lactate dehydrogenase release and terminal deoxynucleotidyl transferase dUTP nick end labeling of targeted doxorubicin micelles in Bcl-2 overexpressing adriamycin-resistant spheroids. Overall, these results support use of spheroids to evaluate tumor targeted drug delivery.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
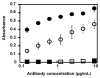
Figure 1. Immunoreactivity of various preparations by indirect ELISA
Filled circles: 2C5 antibody, Circles: 2C5-modified doxorubicin-loaded micelles, Filled squares: Isotype-matched IgG, Squares: IgG-modified doxorubicin-loaded micelles**.** The binding of doxorubicin-loaded PEG-PE micelles harboring 2C5 antibody or isotype antibody to a monolayer of the antigen (nucleohistones) was evaluated by ELISA and detected using an HRP conjugated antibody. Data represent the mean ± SD, n=3.
Figure 2. Uptake of free or micellar doxorubicin by NCI-ADR-RES spheroids
A) NCI-ADR-RES spheroids were either untreated (solid), or incubated 2h at 37 °C with 40μM of free doxorubicin or doxorubicin formulations in media with 7% FBS: free doxorubicin (red), PEG-PE micelles devoid of doxorubicin (black), micellar doxorubicin (green), 2C5-micellar doxorubicin (blue), or isotype-matched micellar doxorubicin (purple). After spheroid dissociation, the cell-associated fluorescence was measured by flow cytometry. B) The geometric mean of MDOX treated spheroids was taken as 100%. Spheroids were individually incubated with formulations before washing with PBS and dissociation with Accumax and repeated pipetting. Ten spheroids per group were pooled for flow cytometry analysis by recording 10,000 gated live cells. **p < 0.01, Student’s t-test compared to free doxorubicin or isotype-matched doxorubicin micelle groups.
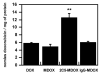
Figure 3. Accumulation of free or micellar doxorubicin in spheroids
NCI-ADR-RES spheroids were incubated 24h in DMEM media with 7% FBS with 40μM of free doxorubicin (DOX), micellar doxorubicin (MDOX), 2C5-micellar doxorubicin (MDOX-2C5) or isotype-matched micellar doxorubicin (MDOX-IgG). Spheroids were individually incubated with doxorubicin formulations before washing with PBS and dispersal in 2% SDS for 1h at 37 °C with pipetting. Doxorubicin accumulation was calculated based on standards in 2% SDS and was expressed in nmoles / mg of protein. Data represent the mean ± SD, n=3. **p < 0.01, Student’s t-test compared to free doxorubicin group.
Figure 4. Penetration of doxorubicin-loaded PEG-PE micelles throughout NCI-ADR-RES spheroids
NCI-ADR-RES spheroids were incubated for 1h at 37 °C with DMEM media 7% FBS containing either HEPES (a) or 40μM of free doxorubicin (b), micellar doxorubicin (c), IgG-MDOX (d) or 2C5-MDOX (e). After the 1h incubation, spheroids were washed with PBS and transferred to Lab-Tek chambers containing complete media. The distribution of doxorubicin was analyzed by confocal microscopy using Z-stack imaging with 20 μm intervals, merges of fluorescence and DIC are shown. Scale bar represents 200 μm.
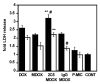
Figure 5. Toxicity of free or doxorubicin-loaded PEG-PE micelles on NCI-ADR-RES spheroids
Spheroids were incubated 48h with 100 μM (black bars) or 50 μM (white bars) of free doxorubicin (DOX), micellar doxorubicin (MDOX), 2C5-MDOX, IgG-MDOX or plain micelles devoid of doxorubicin (P-MIC) before determination of LDH release with a Cytotox 96 cell viability kit. The levels of LDH released in the medium and after lysis of spheroids with 0.9% Triton-X100 were measured. LDH release was normalized to total LDH and was expressed relative to control untreated cells cultured in the same conditions (CONT). LDH release of spheroids treated with micelles devoid of doxorubicin (P-MIC) was not statistically different from untreated spheroids. Data represent the mean ± SD, n=3; **p < 0.01 compared to MDOX-IgG, # p< 0.05 compared to free doxorubicin, Student’s t-test.
Figure 6. Cell death induction by free or micellar doxorubicin on NCI-ADR-RES spheroids
TUNEL assay was performed on untreated spheroids (a), DNAse I treated spheroids (b) or spheroids incubated 48h with 40 μM of free doxorubicin (c), micellar doxorubicin (d), IgG-MDOX (e), 2C5MDOX (f). After inactivation of endogenous peroxidases, fragment end labeling was performed with biotin-labeled deoxynucleotides followed by probing with a peroxidase-streptavidin conjugate and revelation using 3,3′-diaminobenzidine. Scale bar represents 200 μm.
Figure 7. Immunostaining for Bcl-2 on monolayers or spheroids of NCI-ADR-RES cells
A) NCI-ADR-RES cells were grown as monolayers or spheroids and incubated with antibody to Bcl-2 or control isotype-matching antibody. Solid: untreated cells, black line: isotype-matching control on monolayers, blue line: Bcl-2 antibody-treated monolayers, green line: isotype control on spheroids, red line: Bcl-2 antibody-treated spheroids. B) Western blot of lysates from monolayers (M) or spheroids (S) probed for Bcl-2 or beta actin as loading control.
Similar articles
- The effect of co-delivery of paclitaxel and curcumin by transferrin-targeted PEG-PE-based mixed micelles on resistant ovarian cancer in 3-D spheroids and in vivo tumors.
Sarisozen C, Abouzeid AH, Torchilin VP. Sarisozen C, et al. Eur J Pharm Biopharm. 2014 Oct;88(2):539-50. doi: 10.1016/j.ejpb.2014.07.001. Epub 2014 Jul 10. Eur J Pharm Biopharm. 2014. PMID: 25016976 Free PMC article. - Stability influences the biodistribution, toxicity, and anti-tumor activity of doxorubicin encapsulated in PEG-PE micelles in mice.
Wei X, Wang Y, Zeng W, Huang F, Qin L, Zhang C, Liang W. Wei X, et al. Pharm Res. 2012 Jul;29(7):1977-89. doi: 10.1007/s11095-012-0725-5. Epub 2012 Mar 17. Pharm Res. 2012. PMID: 22426964 - Pegylated phosphotidylethanolamine inhibiting P-glycoprotein expression and enhancing retention of doxorubicin in MCF7/ADR cells.
Wang J, Qu H, Jin L, Zeng W, Qin L, Zhang F, Wei X, Lu W, Zhang C, Liang W. Wang J, et al. J Pharm Sci. 2011 Jun;100(6):2267-77. doi: 10.1002/jps.22461. Epub 2011 Jan 18. J Pharm Sci. 2011. PMID: 21246559 - Doxorubicin-mediated radiosensitivity in multicellular spheroids from a lung cancer cell line is enhanced by composite micelle encapsulation.
Xu WH, Han M, Dong Q, Fu ZX, Diao YY, Liu H, Xu J, Jiang HL, Zhang SZ, Zheng S, Gao JQ, Wei QC. Xu WH, et al. Int J Nanomedicine. 2012;7:2661-71. doi: 10.2147/IJN.S30445. Epub 2012 May 29. Int J Nanomedicine. 2012. PMID: 22679376 Free PMC article. - PEG-oligocholic acid telodendrimer micelles for the targeted delivery of doxorubicin to B-cell lymphoma.
Xiao K, Luo J, Li Y, Lee JS, Fung G, Lam KS. Xiao K, et al. J Control Release. 2011 Oct 30;155(2):272-81. doi: 10.1016/j.jconrel.2011.07.018. Epub 2011 Jul 19. J Control Release. 2011. PMID: 21787818 Free PMC article.
Cited by
- Enhanced antitumor efficacy of doxorubicin-encapsulated halloysite nanotubes.
Li K, Zhang Y, Chen M, Hu Y, Jiang W, Zhou L, Li S, Xu M, Zhao Q, Wan R. Li K, et al. Int J Nanomedicine. 2017 Dec 19;13:19-30. doi: 10.2147/IJN.S143928. eCollection 2018. Int J Nanomedicine. 2017. PMID: 29296083 Free PMC article. - pH-Sensitive Multiligand Gold Nanoplatform Targeting Carbonic Anhydrase IX Enhances the Delivery of Doxorubicin to Hypoxic Tumor Spheroids and Overcomes the Hypoxia-Induced Chemoresistance.
Shabana AM, Mondal UK, Alam MR, Spoon T, Ross CA, Madesh M, Supuran CT, Ilies MA. Shabana AM, et al. ACS Appl Mater Interfaces. 2018 May 30;10(21):17792-17808. doi: 10.1021/acsami.8b05607. Epub 2018 May 15. ACS Appl Mater Interfaces. 2018. PMID: 29733576 Free PMC article. - Targeting of Micelles and Liposomes Loaded with the Pro-Apoptotic Drug, NCL-240, into NCI/ADR-RES Cells in a 3D Spheroid Model.
Pattni BS, Nagelli SG, Aryasomayajula B, Deshpande PP, Kulkarni A, Hartner WC, Thakur G, Degterev A, Torchilin VP. Pattni BS, et al. Pharm Res. 2016 Oct;33(10):2540-51. doi: 10.1007/s11095-016-1978-1. Epub 2016 Jun 28. Pharm Res. 2016. PMID: 27351426 Free PMC article. - Cytotoxicity Effects of Curcumin Loaded on Chitosan Alginate Nanospheres on the KMBC-10 Spheroids Cell Line.
Afzali E, Eslaminejad T, Yazdi Rouholamini SE, Shahrokhi-Farjah M, Ansari M. Afzali E, et al. Int J Nanomedicine. 2021 Jan 25;16:579-589. doi: 10.2147/IJN.S251056. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33531802 Free PMC article. - Superior Performance of Aptamer in Tumor Penetration over Antibody: Implication of Aptamer-Based Theranostics in Solid Tumors.
Xiang D, Zheng C, Zhou SF, Qiao S, Tran PH, Pu C, Li Y, Kong L, Kouzani AZ, Lin J, Liu K, Li L, Shigdar S, Duan W. Xiang D, et al. Theranostics. 2015 Jul 2;5(10):1083-97. doi: 10.7150/thno.11711. eCollection 2015. Theranostics. 2015. PMID: 26199647 Free PMC article.
References
- World Health Organization Cancer. 2011. Fact Sheet # 297.
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
- Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, Colombo N, Fowler JM, Argenta PA, De Geest K, Mutch DG, Burger RA, Swart AM, Trimble EL, Accario-Winslow C, Roth LM. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27:1419–1425. - PMC - PubMed
- Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374:1371–1382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources