Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1 - PubMed (original) (raw)
Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1
Liron Bar-Peled et al. Cell. 2012.
Abstract
The mTOR Complex 1 (mTORC1) pathway regulates cell growth in response to numerous cues, including amino acids, which promote mTORC1 translocation to the lysosomal surface, its site of activation. The heterodimeric RagA/B-RagC/D GTPases, the Ragulator complex that tethers the Rags to the lysosome, and the v-ATPase form a signaling system that is necessary for amino acid sensing by mTORC1. Amino acids stimulate the binding of guanosine triphosphate to RagA and RagB but the factors that regulate Rag nucleotide loading are unknown. Here, we identify HBXIP and C7orf59 as two additional Ragulator components that are required for mTORC1 activation by amino acids. The expanded Ragulator has nucleotide exchange activity toward RagA and RagB and interacts with the Rag heterodimers in an amino acid- and v-ATPase-dependent fashion. Thus, we provide mechanistic insight into how mTORC1 senses amino acids by identifying Ragulator as a guanine nucleotide exchange factor (GEF) for the Rag GTPases.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Figure 1. HBXIP and C7orf59 are components of an expanded Ragulator complex
A) Recombinant epitope-tagged HBXIP co-immunoprecipitates endogenous MP1, p18, RagA, and RagC. Anti-FLAG immunoprecipitates were prepared from HEK-293T cells transfected with the indicated cDNAs in expression vectors. Cell lysates and immunoprecipitates were analyzed by immunoblotting for levels of indicated proteins. B) Recombinant C7orf59 co-immunoprecipitates endogenous MP1, p18, RagA, and RagC. HEK-293T cells were transfected with the indicated cDNAs in expression vectors and analyzed as in (A). C) Endogenous p18 co-immunoprecipitates endogenous p14, MP1, RagA, RagC, HBXIP, and C7orf59. Anti-p18 immunoprecipitates were prepared from HEK-293T cells and analyzed for the levels of the indicated proteins. D) Images of HEK-293T cells co-immunostained for p18 (green) and FLAG-HBXIP (red) or FLAG-C7orf59 (red). Cells were co-transfected with cDNAs encoding MP1, p14, and p18 and either FLAG-HBXIP or FLAG-C7orf59 and processed for immunostaining and imaging. In all images, insets show selected fields that were magnified five times and their overlays. Scale bar represents 10 μm. E) Ragulator is a pentameric complex. In vitro binding assay in which recombinant HA-GST-tagged-p14-MP1, -C7orf59 or -HBXIP were incubated with the indicated purified FLAG-tagged Ragulator proteins. HA-GST precipitates were analyzed for levels of the indicated proteins. F) Schematic summarizing intra-Ragulator interactions: p18 bridges MP1-p14 with HBXIP-C7orf59. G) The pentameric Ragulator complex co-immunoprecipitates recombinant RagB and RagC. HEK-293T cells were co-transfected with the indicated cDNAs in expression vectors and analyzed as in (A). H) Requirement for a pentameric Ragulator complex to interact with Rags. In vitro binding assay in which recombinant HA-GST Ragulator with or without p18 was incubated with purified FLAG-RagB-RagC and analyzed as in (E). See also Figure S1.
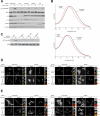
Figure 2. HBXIP and C7orf59 are necessary for TORC1 activation by amino acids and localization of the Rag GTPases and mTOR to the lysosomal surface
A) C7orf59 and HBXIP are necessary for the activation of the mTORC1 pathway by amino acids. HEK-293T cells, treated with siRNAs targeting the mRNAs for the indicated proteins, were starved of amino acids for 50 min, or starved and stimulated with amino acids for 10 min. Immunoblot analyses were used to measure the levels of the indicated proteins and phosphorylation states. B) Cells depleted of C7orf59 and HBXIP are smaller than controls. Cell size distributions are shown for HEK-293T cells treated with siRNAs targeting C7orf59, HBXIP, or a scrambled non-targeting control. C) Drosophila orthologs of HBXIP and C7orf59 are required for the activation of the TORC1 pathway. Drosophila S2 cells were transfected with a control dsRNA or dsRNAs targeting dRagC, dC7orf59, or dHBXIP, starved for amino acids for 90 min or starved and stimulated with amino acids for 30 min and analyzed as in (A). D) Images of HEK-293T cells, treated with a non-targeting siRNA or siRNAs targeting HBXIP or C7orf59, co-immunostained for RagA (red) and LAMP2 (green). Cells were starved for amino acids or starved and stimulated for the indicated times before processing for the immunofluorescence assay and imaging. E) Images of HEK-293T cells, treated with a non-targeting siRNA or siRNAs targeting HBXIP or C7orf59, co-immunostained for mTOR (red) and LAMP2 (green). Cells were treated and processed as in (A). In all images, insets show selected fields that were magnified five times and their overlays. Scale bars represent 10 μm. See also Figure S2.
Figure 3. Amino acids regulate the Rag-Ragulator interaction
A) Amino acids regulate the amounts of endogenous RagA and RagC that co-immunoprecipitate with recombinant p14 and p18. HEK-293T cells, transfected with the indicated cDNAs in expression vectors, were starved for amino acids for 2 hours or starved and stimulated with amino acids for 15 min. Anti-FLAG immunoprecipitates were prepared from cell lysates and analyzed by immunoblotting for levels of indicated proteins. B) Amino acid starvation strengthens the interaction between endogenous Rags and recombinant C7orf59 and HBXIP. HEK-293T cells stably expressing FLAG-C7orf59 or FLAG-HBXIP were starved and re-stimulated with amino acids. Anti-FLAG immunoprecipitates were analyzed as in (A). C) FLAG-RagB co-immunoprecipitation of endogenous Ragulator is dependent on amino acids. HEK-293T cells stably expressing FLAG-RagB were starved and re-stimulated with amino acids as in (A). Anti-FLAG immunoprecipitates were analyzed for the levels of the indicated proteins. D) Leucine starvation strengthens the binding of Ragulator to endogenous Rags and the V1 domain of the v-ATPase. HEK-293T cells stably expressing FLAG-p18 were starved and re-stimulated with total amino acids as in (A) or starved for leucine for 2 hours or starved and stimulated with leucine for 20 min. Anti-FLAG immunoprecipitates were analyzed for the levels of the indicated proteins. See also Figure S3.
Figure 4. Ragulator preferentially interacts with nucleotide-free RagB
A) EDTA increases the interaction between endogenous Ragulator and FLAG-RagB. HEK-293T cells stably expressing Flag-RagB were lysed in the absence or presence of EDTA and cell lysates and anti-FLAG immunoprecipitates analyzed by immunoblotting for the levels of the indicated proteins. B) FLAG-p18 co-immunoprecipitates more endogenous Rags in the presence of EDTA. HEK-293T cells stably expressing FLAG-p18 were treated and analyzed as in (A). C) Ragulator preferentially interacts with nucleotide-free Rags. In vitro binding assay in which immobilized HA-GST-Ragulator was incubated with nucleotide-free FLAG-RagB-RagC or Rag heterodimers loaded with GTP or GDP. HA-GST precipitates were analyzed for the levels of the indicated proteins. D) Table summarizing Rag mutants used in this study. E) The RagBT54N mutant preferentially interacts with endogenous Ragulator. Anti-FLAG immunoprecipitates were prepared from HEK-293T cells transfected with the indicated cDNAs in expression vectors and analyzed as in (A). F) Quantification of endogenous MP1 and p18 binding to RagBT54N-RagC and RagBRagCS75N. Each value represents the normalized mean ±SD for n=3. See also Figure S4.
Figure 5. Ragulator is a GEF for RagA and RagB
A) Ragulator does not stimulate GDP or GTPγS dissociation from Rap2a. Nucleotide dissociation assay, in which Rap2a was loaded with either [3H]GDP or [35S]GTPγS, and incubated with Ragulator. Dissociation was monitored by a filter-binding assay. Each value represents the normalized mean ±SD for n=4. B) Ragulator moderately stimulates GDP, but not GTPγS dissociation from RagC. RagBD163N-RagC was loaded, incubated with Ragulator or a control and analyzed as in (A). Each value represents the normalized mean ±SD for n=4. C) Ragulator greatly accelerates GDP and GTPγS dissociation from RagB. RagB-RagCD181N was loaded, incubated with Ragulator or a control and analyzed as in (A). Each value represents the normalized mean ±SD for n=4. D) Ragulator substantially increases GDP and GTPγS dissociation from RagA. RagA-RagCD181N was loaded with nucleotide, incubated with Ragulator or a control and analyzed as in (A). Each value represents the normalized mean ±SD for n=4. E) Ragulator accelerates GTPγS binding to RagB. RagB-RagCD181N was loaded with GDP and incubated with Ragulator or a control and [35S]GTPγS. [35S]GTPγS binding was determined as in (A). Each value represents the normalized mean ±SD for n=4. F) Ragulator potentiates GTPγS binding to RagA. RagA-RagCD181N was loaded with GDP and incubated with Ragulator or a control and [35S]GTPγS. [35S]GTPγS binding was determined as in (A). Each value represents the normalized mean ±SD for n=4. G) Individual Ragulator subunits do not increase GDP dissociation from RagB. [3H]GDP bound RagB-RagCD181N was incubated with Ragulator, p14-MP1, HBXIP-C7orf59, or p18, and [3H]GDP dissociation monitored as in (A). Each value represents the normalized mean ±SD for n=4. H) Trimeric Ragulator complexes do not increase GTPγS dissociation from RagB. [35S]GTPγS bound RagB-CD181N was incubated with Ragulator, p14-MP1-p18, or HBXIP-C7orf59-p18 and dissociation was monitored as in (A). Each value represents the normalized mean ±SD for n=4. See also Figure S5.
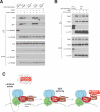
Figure 6. The v-ATPase controls the function of Ragulator
A) The v-ATPase functions upstream of the regulated binding between Rags and Ragulator. HEK-293T cells stably expressing FLAG-RagB or FLAG-RagBQ99L were starved for 2 hours or starved and stimulated with amino acids for 15 min in the absence or presence of the v-ATPase inhibitor SalA. Cell lysates and anti-FLAG immunoprecipitates were analyzed by immunoblotting for the levels of the indicated proteins. B) Inactivation of the v-ATPase blocks the amino acid dependent Rag-Ragulator interaction. HEK-293T cells stably expressing FLAG-p18 were treated as in (B). Anti-FLAG immunoprecipitates were analyzed for the levels of the indicated proteins. C) Model for amino-acid induced mTORC1 activation. In the absence of amino acids the v-ATPase, Ragulator, and Rags exist in a tightly bound super complex and mTORC1 cannot associate with the lysosomal surface and remains inactive. Amino acid accumulation in the lysosomal lumen generates an activating signal that is transmitted in a v-ATPase-dependent fashion to activate the GEF activity of Ragulator towards RagA. Upon RagA-GTP loading, the Rag-Ragulator interaction weakens and mTORC1 is recruited to the lysosomal surface where it interacts with Rheb and becomes activated. Rheb is not shown.
Similar articles
- Ragulator and SLC38A9 activate the Rag GTPases through noncanonical GEF mechanisms.
Shen K, Sabatini DM. Shen K, et al. Proc Natl Acad Sci U S A. 2018 Sep 18;115(38):9545-9550. doi: 10.1073/pnas.1811727115. Epub 2018 Sep 4. Proc Natl Acad Sci U S A. 2018. PMID: 30181260 Free PMC article. - Amino acids activate mammalian target of rapamycin (mTOR) complex 1 without changing Rag GTPase guanyl nucleotide charging.
Oshiro N, Rapley J, Avruch J. Oshiro N, et al. J Biol Chem. 2014 Jan 31;289(5):2658-74. doi: 10.1074/jbc.M113.528505. Epub 2013 Dec 11. J Biol Chem. 2014. PMID: 24337580 Free PMC article. - Rag-Ragulator is the central organizer of the physical architecture of the mTORC1 nutrient-sensing pathway.
Valenstein ML, Lalgudi PV, Gu X, Kedir JF, Taylor MS, Chivukula RR, Sabatini DM. Valenstein ML, et al. Proc Natl Acad Sci U S A. 2024 Aug 27;121(35):e2322755121. doi: 10.1073/pnas.2322755121. Epub 2024 Aug 20. Proc Natl Acad Sci U S A. 2024. PMID: 39163330 - Rag GTPase in amino acid signaling.
Kim J, Kim E. Kim J, et al. Amino Acids. 2016 Apr;48(4):915-928. doi: 10.1007/s00726-016-2171-x. Epub 2016 Jan 18. Amino Acids. 2016. PMID: 26781224 Review. - Regulation of mTORC1 by amino acids.
Bar-Peled L, Sabatini DM. Bar-Peled L, et al. Trends Cell Biol. 2014 Jul;24(7):400-6. doi: 10.1016/j.tcb.2014.03.003. Epub 2014 Mar 31. Trends Cell Biol. 2014. PMID: 24698685 Free PMC article. Review.
Cited by
- A lysosome-centered view of nutrient homeostasis.
Mony VK, Benjamin S, O'Rourke EJ. Mony VK, et al. Autophagy. 2016;12(4):619-31. doi: 10.1080/15548627.2016.1147671. Autophagy. 2016. PMID: 27050453 Free PMC article. Review. - Sorafenib triggers ferroptosis via inhibition of HBXIP/SCD axis in hepatocellular carcinoma.
Zhang L, Li XM, Shi XH, Ye K, Fu XL, Wang X, Guo SM, Ma JQ, Xu FF, Sun HM, Li QQ, Zhang WY, Ye LH. Zhang L, et al. Acta Pharmacol Sin. 2023 Mar;44(3):622-634. doi: 10.1038/s41401-022-00981-9. Epub 2022 Sep 15. Acta Pharmacol Sin. 2023. PMID: 36109580 Free PMC article. - SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1.
Rebsamen M, Pochini L, Stasyk T, de Araújo ME, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M, Scorzoni S, Filipek PA, Huber KV, Bigenzahn JW, Heinz LX, Kraft C, Bennett KL, Indiveri C, Huber LA, Superti-Furga G. Rebsamen M, et al. Nature. 2015 Mar 26;519(7544):477-81. doi: 10.1038/nature14107. Epub 2015 Jan 7. Nature. 2015. PMID: 25561175 Free PMC article. - The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging.
Kennedy BK, Lamming DW. Kennedy BK, et al. Cell Metab. 2016 Jun 14;23(6):990-1003. doi: 10.1016/j.cmet.2016.05.009. Cell Metab. 2016. PMID: 27304501 Free PMC article. Review. - Targeting cellular adaptive responses to glutaminolysis perturbation for cancer therapy.
Kim M, Hwang S, Jeong SM. Kim M, et al. Mol Cells. 2024 Aug;47(8):100096. doi: 10.1016/j.mocell.2024.100096. Epub 2024 Jul 20. Mol Cells. 2024. PMID: 39038517 Free PMC article. Review.
References
- Binda M, PÈli-Gulli M-P, Bonfils G, Panchaud N, Urban J, Sturgill TW, Loewith R, De Virgilio C. The Vam6 GEF Controls TORC1 by Activating the EGO Complex. Mol Cell. 2009;35:563–573. - PubMed
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. - PubMed
- Bowman EJ, Graham LA, Stevens TH, Bowman BJ. The bafilomycin/concanamycin binding site in subunit c of the V-ATPases from Neurospora crassa and Saccharomyces cerevisiae. J Biol Chem. 2004;279:33131–33138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31CA167872/CA/NCI NIH HHS/United States
- F31 CA167872/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- CA103866/CA/NCI NIH HHS/United States
- R37 AI047389/AI/NIAID NIH HHS/United States
- R01 CA129105/CA/NCI NIH HHS/United States
- AI47389/AI/NIAID NIH HHS/United States
- R01 AI047389/AI/NIAID NIH HHS/United States
- R01 CA103866/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous