Prefrontal cortex modulates desire and dread generated by nucleus accumbens glutamate disruption - PubMed (original) (raw)
Prefrontal cortex modulates desire and dread generated by nucleus accumbens glutamate disruption
Jocelyn M Richard et al. Biol Psychiatry. 2013.
Abstract
Background: Corticolimbic circuits, including direct projections from prefrontal cortex to nucleus accumbens (NAc), permit top-down control of intense motivations generated by subcortical circuits. In rats, localized disruptions of glutamate signaling within medial shell of NAc generate desire or dread, anatomically organized along a rostrocaudal gradient analogous to a limbic keyboard. At rostral locations in shell, these disruptions generate appetitive eating, but at caudal locations the disruptions generate progressively fearful behaviors (distress vocalizations, escape attempts, and antipredator reactions). Here, we asked whether medial prefrontal cortex can modulate intense motivations generated by subcortical NAc disruptions.
Methods: We used simultaneous microinjections in medial prefrontal cortex regions and in NAc shell to examine whether the desire or dread generated by NAc shell disruptions is modulated by activation/inhibition of three specific regions of prefrontal cortex: medial orbitofrontal cortex, infralimbic cortex (homologous to area 25 or subgenual anterior cingulate in the human), or prelimbic cortex (midventral anterior cingulate).
Results: We found that activation of medial orbitofrontal cortex biased intense bivalent motivation in an appetitive direction by amplifying generation of eating behavior by middle to caudal NAc disruptions, without altering fear. In contrast, activation of infralimbic prefrontal cortex powerfully and generally suppressed both appetitive eating and fearful behaviors generated by NAc shell disruptions.
Conclusions: These results suggest that corticolimbic projections from discrete prefrontal regions can either bias motivational valence or generally suppress subcortically generated intense motivations of desire or fear.
Copyright © 2013 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Figures
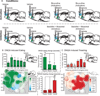
Figure 1. Microinjection conditions and DNQX effects
Rats received the following microinjections (A): rats in the activation group (top, n=68) received either DNQX or vehicle in NAc shell and bicuculline or vehicle into prefrontal cortex, and rats in the inactivation group (bottom, n=30) received either DNQX or vehicle in NAc shell and a baclofen plus muscimol combination or vehicle in prefrontal cortex. Fos plume maps show the effects of DNQX alone (vehicle in prefrontal cortex) on eating (B, green) or defensive treading behavior (C, red). Histogram bars above the maps show mean behaviors as a percent of vehicle at each rostrocaudal level (errors bars = SEM). Summary bar graphs show the DNQX induced eating (B) and treading (C) as change from vehicle at rostral (n=26), middle (n=30) and caudal (n=22) locations in NAc shell; data is given as seconds per hour, ** p < .01, * p < .05 versus vehicle, ## p < .01, # p < .05 subregion difference, with Sidak corrections for multiple comparisons.
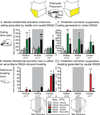
Figure 2. Maps of prefrontal activation effects on NAc shell DNQX generated eating and defensive treading
Maps show the effects of prefrontal activation (n=68) on DNQX-induced eating (A, left) or defensive treading (B, right) at sites mapped on the sagittal plane of prefrontal cortex, color-coded for changes in behavior as a percent of DNQX. Histograms bars show mean behavior as percent of DNQX at each rostrocaudal level, split by dorsal (prelimbic, top; n=11) and ventral (medial orbitofrontal, n=29, and infralimbic, n=26, bottom) areas of prefrontal cortex (error bars = SEM).
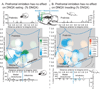
Figure 3. Motivated behavior graphs
Graphs demonstrating the specific effects of medial orbitofrontal activation (left) and infralimbic activation (right) on appetitive eating (top) and defensive treading (bottom), depending on particular rostrocaudal location (rostral, middle or caudal). Simultaneous microinjections of bicuculline in medial orbitofrontal (n=29) with DNQX in NAc shell (black, left) produced enhancement of DNQX induced eating (green, A), specifically at more middle (n=11) and caudal (n=7) locations but not rostral (n=11), and had no effect on DNQX induced treading (red, B). Microinjections of bicuculline in infralimbic cortex (n=26) with simultaneous DNQX in NAc shell (black, right) produced suppression of both DNQX-induced eating (green) from rostral sites (n=12) and treading (red) from caudal sites (n=7; middle sites, n=7). Data is given as seconds per hour, errors bars indicate SEM, * p < .05 versus vehicle, ** p < .01 versus vehicle, # p < .05 versus DNQX, ## p < .01 versus DNQX, pairwise comparison using Sidak corrections.
Figure 4. Maps of prefrontal inhibition effects on NAc shell DNQX generated eating and defensive treading
Maps show the effects of prefrontal inhibition (n=30) on DNQX-induced eating (A, left) or defensive treading (B, right) at sites mapped on the sagittal plane of prefrontal cortex, color-coded for changes in behavior as a percent of DNQX. Histograms bars show mean behavior as percent of DNQX at each rostrocaudal level, split by dorsal (prelimbic, top; n=5) and ventral (medial orbitofrontal, n=16, and infralimbic, n=9, bottom) areas of prefrontal cortex (error bars = SEM).
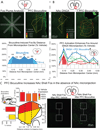
Figure 5. Fos plume analysis and NAc-prefrontal interactions
Fos plumes were analyzed for functional drug spread of bicuculline in prefrontal cortex (A) and DNQX in NAc shell (B). Fos labeled cells were individually counted within successive blocks (50 µm×50 µm), along 8 radial arms emanating from the center of the site, with 10x magnification (A). Colors indicate levels of Fos expression of 3x (red), 2x (orange) and 1.5x (yellow) vehicle level Fos expression. Line graphs show levels of Fos expression following bicuculline (blue, A) and DNQX (red, B), as well as the impact of prefrontal bicuculline on levels of Fos expression in NAc shell following either DNQX (blue, B) or vehicle (green, B) microinjections in NAc shell. Analysis of Fos expression in uninjected NAc shell (C) showed that bicuculline elevated NAc shell Fos even in the absence of NAc shell microinjections at levels more than 500% (yellow), 650% (orange), and 800% (red) of vehicle. Bar graphs indicate levels of elevated Fos at three rostrocaudal and two dorsoventral levels. * p < .05, ** p < .01 versus vehicle, # p < .05, ## p < .01 versus DNQX.
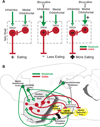
Figure 6. Potential mechanisms of prefrontal modulation of DNQX generated motivated behaviors
Proposed direct prefrontal to NAc shell mechanism (A) mediating opposite infralimbic versus orbitofrontal effects on DNQX-induced eating. Bicuculline infusions excite glutamate inputs (green) from infralimbic or medial orbitofrontal cortex. DNQX alone inhibits rostral NAc shell projection neurons (red), resulting in disinhibition of downstream targets and intense eating. Infralimbic activation may overcome DNQX inhibition of these same neurons, suppressing eating. Medial orbitofrontal activation may instead activate GABAergic interneurons, which further inhibit neurons already inhibited by DNQX, potentiating eating. A circuit diagram (B) shows prefrontal and NAc shell projections to relevant third-party structures that may mediate larger circuit interactions. DNQX microinjections likely inhibit neurons in NAc shell, disinhibiting downstream structures such as ventral pallidum, lateral hypothalamus, and ventral tegmental area (yellow) via GABAergic projections neurons (red). Medial orbitofrontal and infralimbic activation may act to modulate DNQX-induced behaviors via direct glutamate (green) projections to NAc shell or parallel projections to structures such as medial dorsal hypothalamus, lateral hypothalamus and basolateral amygdala.
Similar articles
- Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D(1) alone for appetitive eating but D(1) and D(2) together for fear.
Richard JM, Berridge KC. Richard JM, et al. J Neurosci. 2011 Sep 7;31(36):12866-79. doi: 10.1523/JNEUROSCI.1339-11.2011. J Neurosci. 2011. PMID: 21900565 Free PMC article. - Desire or Dread from Nucleus Accumbens Inhibitions: Reversed by Same-Site Optogenetic Excitations.
Baumgartner HM, Cole SL, Olney JJ, Berridge KC. Baumgartner HM, et al. J Neurosci. 2020 Mar 25;40(13):2737-2752. doi: 10.1523/JNEUROSCI.2902-19.2020. Epub 2020 Feb 19. J Neurosci. 2020. PMID: 32075899 Free PMC article. - Desire and dread from the nucleus accumbens: cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat.
Faure A, Richard JM, Berridge KC. Faure A, et al. PLoS One. 2010 Jun 18;5(6):e11223. doi: 10.1371/journal.pone.0011223. PLoS One. 2010. PMID: 20585461 Free PMC article. - Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex.
Del Arco A, Mora F. Del Arco A, et al. Pharmacol Biochem Behav. 2008 Aug;90(2):226-35. doi: 10.1016/j.pbb.2008.04.011. Epub 2008 Apr 23. Pharmacol Biochem Behav. 2008. PMID: 18508116 Review. - Feeding-modulatory effects of mu-opioids in the medial prefrontal cortex: a review of recent findings and comparison to opioid actions in the nucleus accumbens.
Selleck RA, Baldo BA. Selleck RA, et al. Psychopharmacology (Berl). 2017 May;234(9-10):1439-1449. doi: 10.1007/s00213-016-4522-4. Epub 2017 Jan 4. Psychopharmacology (Berl). 2017. PMID: 28054099 Free PMC article. Review.
Cited by
- Direct hypothalamic and indirect trans-pallidal, trans-thalamic, or trans-septal control of accumbens signaling and their roles in food intake.
Urstadt KR, Stanley BG. Urstadt KR, et al. Front Syst Neurosci. 2015 Feb 13;9:8. doi: 10.3389/fnsys.2015.00008. eCollection 2015. Front Syst Neurosci. 2015. PMID: 25741246 Free PMC article. Review. - Contemporary approaches to neural circuit manipulation and mapping: focus on reward and addiction.
Saunders BT, Richard JM, Janak PH. Saunders BT, et al. Philos Trans R Soc Lond B Biol Sci. 2015 Sep 19;370(1677):20140210. doi: 10.1098/rstb.2014.0210. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26240425 Free PMC article. Review. - Revisiting the role of infralimbic cortex in fear extinction with optogenetics.
Do-Monte FH, Manzano-Nieves G, Quiñones-Laracuente K, Ramos-Medina L, Quirk GJ. Do-Monte FH, et al. J Neurosci. 2015 Feb 25;35(8):3607-15. doi: 10.1523/JNEUROSCI.3137-14.2015. J Neurosci. 2015. PMID: 25716859 Free PMC article. - Local Control of Extracellular Dopamine Levels in the Medial Nucleus Accumbens by a Glutamatergic Projection from the Infralimbic Cortex.
Quiroz C, Orrú M, Rea W, Ciudad-Roberts A, Yepes G, Britt JP, Ferré S. Quiroz C, et al. J Neurosci. 2016 Jan 20;36(3):851-9. doi: 10.1523/JNEUROSCI.2850-15.2016. J Neurosci. 2016. PMID: 26791215 Free PMC article. - Involvement of the rodent prelimbic and medial orbitofrontal cortices in goal-directed action: A brief review.
Woon EP, Sequeira MK, Barbee BR, Gourley SL. Woon EP, et al. J Neurosci Res. 2020 Jun;98(6):1020-1030. doi: 10.1002/jnr.24567. Epub 2019 Dec 10. J Neurosci Res. 2020. PMID: 31820488 Free PMC article. Review.
References
- Kompus K, Hugdahl K, Ohman A, Marklund P, Nyberg L. Distinct control networks for cognition and emotion in the prefrontal cortex. Neurosci Lett. 2009;467:76–80. - PubMed
- Phillips AG, Vacca G, Ahn S. A top-down perspective on dopamine, motivation and memory. Pharmacology Biochemistry and Behavior. 2008;90:236–249. - PubMed
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte M, Jansen A. Fighting food temptations: The modulating effects of short-term cognitive reappraisal, suppression and up-regulation on mesocorticolimbic activity related to appetitive motivation. Neuroimage. 2012;60:213–220. - PubMed
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH090602/MH/NIMH NIH HHS/United States
- MH63649/MH/NIMH NIH HHS/United States
- R01 MH063649/MH/NIMH NIH HHS/United States
- R01 DA015188/DA/NIDA NIH HHS/United States
- DA015188/DA/NIDA NIH HHS/United States
- F31 MH090602/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources