Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling - PubMed (original) (raw)
. 2012 Oct;15(10):1399-406.
doi: 10.1038/nn.3212. Epub 2012 Sep 16.
Affiliations
- PMID: 22983209
- PMCID: PMC3458152
- DOI: 10.1038/nn.3212
Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling
Randolph S Ashton et al. Nat Neurosci. 2012 Oct.
Abstract
Neurogenesis in the adult hippocampus involves activation of quiescent neural stem cells (NSCs) to yield transiently amplifying NSCs, progenitors, and, ultimately, neurons that affect learning and memory. This process is tightly controlled by microenvironmental cues, although a few endogenous factors are known to regulate neuronal differentiation. Astrocytes have been implicated, but their role in juxtacrine (that is, cell-cell contact dependent) signaling in NSC niches has not been investigated. We found that ephrin-B2 presented from rodent hippocampal astrocytes regulated neurogenesis in vivo. Furthermore, clonal analysis in NSC fate-mapping studies revealed a previously unknown role for ephrin-B2 in instructing neuronal differentiation. In addition, ephrin-B2 signaling, transduced by EphB4 receptors on NSCs, activated β-catenin in vitro and in vivo independently of Wnt signaling and upregulated proneural transcription factors. Ephrin-B2(+) astrocytes therefore promote neuronal differentiation of adult NSCs through juxtacrine signaling, findings that advance our understanding of adult neurogenesis and may have future regenerative medicine implications.
Figures
Figure 1
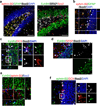
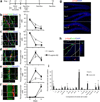
In vivo, SGZ Type 2a NSCs, Type 2b neuronal precursors, and Type 3 neuroblasts express EphB4, and hippocampal astrocytes express ephrin-B2. (a) Staining of the hippocampal dentate gyrus showed that GFAP+ hilar (H) astrocytes express ephrin-B2. In addition, cells in the SGZ and neurons in the granule cell layer (GCL) express EphB4+ on the cell soma. Scale bar represents 100 µm. (b) GFAP+ astrocytes adjacent to the SGZ co-express ephrin-B2. (c,d) EphB4 expression persists throughout NSC neuronal differentiation, including Type 2a NSCs (Sox2+/DCX−/GFAP−), Type 2b neuronal precursors (Sox2+/DCX+), and Type 3 neuroblasts (DCX+). (c) Confocal images show EphB4 expression in Sox2+/DCX− cells (large arrowheads) in the SGZ. Magnified region depicts the presence of EphB4 expression on a Sox2+/DCX+ Type 2b neuronal precursor (small arrowhead) and a DCX+ Type 3 neuroblast (arrow). (d) Confocal images also show EphB4 expression by Sox2+/GFAP− cells (small arrowheads); therefore, Sox2+/DCX−/GFAP− Type 2a NSCs also express EphB4. (e) Given the close proximity of ephrin-B2+ astrocytes to EphB4+ cells in the SGZ, ephrin-B2/EphB4 juxtacrine signaling is in a position to induce NSC differentiation into (f) Sox2+/DCX+ Type 2b neuronal precursors (small arrowhead) and subsequently Sox2−/DCX+ Type 3 neuroblasts (arrow). The scale bars represent 10 µm.
Figure 2
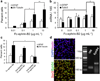
Fc-ephrin-B2 promoted the neuronal differentiation of NSCs in vitro. (a,b) Stimulation by Fc-Ephrin-B2 induced NSCs to undergo neuronal differentiation (βIII-Tubulin+ or Tubb3) in a dose-responsive fashion as measured by immunocytochemistry and QPCR (n = 3, experimental replicates). Glial fibrilary acidic protein (GFAP) staining was slightly increased with ephrin-B2, but no increase in expression was observed by QPCR. (c) Blockage of ephrin-B2 receptors, EphB2 and EphB4, during Fc-ephrin-B2 (10 µg/mL) stimulation revealed that EphB4 mediates Fc-ephrin-B2’s proneuronal effect on NSCs (n = 3, experimental replicates). ANOVA plus a multi-variable Tukey-Kramer analysis was conducted, with * indicating P <0.01 and ** indicating P <0.05. Data are represented as means ± s.d. (d) Nestin+/Sox2+ NSCs express EphB4 as demonstrated by immunocytochemistry and RT-PCR. Scale bar represents 100 µm.
Figure 3
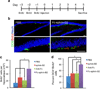
Intrahippocampal injection of Fc-ephrin-B2 increases neurogenesis in the SGZ. (a) Schematic of experimental time course. (b) Administration of Fc-ephrin-B2 into the hippocampus significantly increased neurogenesis as shown by representative confocal images of the dentate gyrus and SGZ (close-up). The scale bar represents 100 µm. (c) The injection of exogenous ephrin-B2 ligands increased BrdU+ cell numbers compared to vehicle and constituent controls. (d) Only injection of Fc-ephrin-B2, but not its constituent controls, increased the percentage of BrdU+ cells that co-stained for DCX in the SGZ (n = 3 brains, analyzed 8 hippocampal sections per brain). * indicates P <0.01, and ** indicates P <0.05; ± s.d.
Figure 4
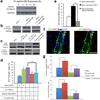
Ephrin-B2 RNAi decreases the proneuronal effect of hippocampus-derived astrocytes in vitro. (a) Efnb2 shRNA lentiviral vectors (#1 and #2) significantly inhibit efnb2 expression in hippocampus-derived astrocytes. Expression levels were measured by QPCR and normalized to efnb2 expression in non-infected hippocampus-derived astrocytes (i.e. 100%). Also, hippocampus-derived astrocytes express orders of magnitude more efnb2 than NSCs (n = 3, technical replicates). (b) Naïve astrocytes and astrocytes expressing a control LacZ shRNA promoted neuronal differentiation of NSCs, compared to NSC-only cultures. However, knockdown of astrocyte efnb2 expression, or antibody blockage of NSC EphB4 receptors, significantly diminished the proneuronal effect of hippocampus-derived astrocytes, as demonstrated by the decrease in the percentage of βIII-Tubulin+/ BrdU+ NSCs to levels closer to those in NSC-only cultures (n = 4, experimental replicates). The solid black line indicates the level of βIII-Tubulin+/BrdU+ cells at the start of the experiment. (c) Representative confocal image of BrdU-labeled NSCs differentiated into βIII-Tubulin+ neurons after co-culture with lentiviral vector-expressing, i.e. GFP+, astrocytes. NSCs adjacent to astrocytes had a higher propensity for neuronal differentiation. Scale bar represents 100 µm. * indicates P <0.01; ± s.d.
Figure 5
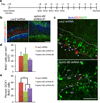
Ephrin-B2 RNAi decreases neuronal differentiation of BrdU+ cells in the SGZ. (a) Schematic of experimental time course. (b) Regions of the hippocampus transduced with lentiviral vector (GFP+) carrying efnb2 shRNA #1 and #2 (data not shown) showed considerably less ephrin-B2 staining than hippocampi transduced with LacZ shRNA lentivirus injected rats. (c) Representative confocal images showing decreased neuronal differentiation, i.e. DCX+ co-staining, of BrdU+ (arrowheads) cells in the SGZ of rats injected with lentivirus encoding efnb2 shRNA. Scale bars represent 100 µm. (d,e) Knockdown of ephrin-B2 in the hippocampal niche did not affect BrdU+ cell numbers, but it did result in a significant decrease in the percentage of BrdU+ cells that co-stained for DCX in the SGZ (n = 4 brains, analyzed 8 hippocampal sections per brain). This suggests that endogenous ephrin-B2 signaling regulates neuronal differentiation of NSCs. * indicates p<0.01; ± s.d; dotted line marks SGZ/Hilus boundary and dashed line marks GCL/MCL boundary.
Figure 6
Lineage tracing of ephrin-B2-induced NSC differentiation. (a) Time course where (b,c) initially 90.7 ± 1.79% of β-Gal+ cells were Nestin+/Sox2+, and 53.8 ± 5.49% were GFAP+ along radial process (Type 1 NSCs). (d,e) By day 5, 38.7 ± 2.73% and 37.3 ± 4.51% of β-Gal+ cells were Sox2+/DCX+ and DCX+/NeuroD1+, respectively, in Fc-ephrin-B2 injected mice vs. 27.7 ± 5.16% and 13.8 ± 4.38% in Anti-Fc controls. (f) At day 14, 41.1 ± 4.86% of β-Gal+ cells were NeuN+ for Fc-ephrin-B2 vs. 25.6 ± 4.24% for controls. (g,h) Representative sections where β-Gal+ and BrdU+ cells proliferated and differentiated over 14 days. (i) Compared to Anti-Fc, Fc-ephrin-B2 decreased the number of single Type 1 (“1”, 8.01 ± 1.91% vs. 17.8 ± 1.93%) and Type 2a NSCs (“2a”, 2.51 ± 2.34% vs. 10.8 ± 3.22%) and increased single neuroblast or neurons (“N”, 49.2 ± 4.57% vs. 30.9 ± 2.28%). Furthermore, ephrin-B2 decreased the number of doublets containing a Type 1 (“1+X”, 5.95 ± 1.47% vs. 11.8 ± 2.03%) or Type 2 (“2a+X”, 7.37 ± 3.00% vs. 16.5 ± 6.03%) cell and increased neuroblasts or neuron doublets (“N+N”,16.3 ± 1.82% vs. 3.87 ± 1.65%). Cluster size (1.55 ± 0.03 vs. 1.54 ± 0.04) and overall β-Gal+ cell numbers (Supplementary Fig. 5) were indistinguishable in Fc-ephrin-B2 vs. control mice. Thus, ephrin-B2 signaling increases neuronal differentiation without altering proliferation. ** P <0.05; ± s.d; Five sections (10 hemispheres) analyzed in n = 4 Anti-Fc and 5 Fc-ephrin-B2 brains; dotted vs. dashed lines mark SGZ/Hilus vs. GCL/MCL boundaries.
Figure 7
Ephrin-B2 instructs neuronal differentiation by activating β-catenin independent of Wnt signaling. (a) Fc-ephrin-B2 (10 µg/mL) induced active β-catenin accumulation in NSCs over a 24 hours. (b) However, blocking the EphB4 receptor compromised Fc-ephrin-B2 induction of β-catenin accumulation. (c) NSCs expressing a constitutively active GSK3 β, GSK3 ββS9A, did not accumulate β-catenin in response to Fc-ephrin-B2 (10 µg/mL for 24 hours), in contrast to naïve or empty vector control NSCs (CTL NSCs). (d) Constitutive degradation of β-catenin in GSK3 βS9A NSCs decreased NSCs differentiation into βIII-Tubulin+ neurons in response to Fc-ephrin-B2 (10 µg/mL) vs. empty vector control NPCs (CTL NPCs) (n = 3 experimental replicates). (e) The lack of β-catenin signaling in GSK3 βS9A NSCs also nullified the proneuronal effect of hippocampus-derived, ephrin-B2 expressing astrocytes in co-culture (n=4, experimental repeats). (f) In mice co-infected with Tcf-Luc and dnWnt-IRES-GFP constructs, cells in the SGZ still expressed active β-catenin (ABC) and Luciferase (arrowheads) 24 hours after Fc-ephrin-B2 injection. Scale bar represents 100 µm. (g) In hippocampi co-infected with Tcf-Luc and either dnWnt-IRES-GFP or IRES-GFP construct, then injected with Fc-ephrin-B2 or PBS, Fc-ephrin-B2 increased the percentage of SGZ BrdU+ cells with active β-catenin signaling 24 hours post-injection and the percentage of DCX+/BrdU+ cells by day 5 even with dnWnt present (n=4 brains, 8 sections per brain). Ephrin-B2 thus activates β-catenin signaling and enhances adult neurogenesis independent of Wnt signaling. * indicates P <0.01; ** indicates P <0.05; ± s.d; dotted vs. dashed lines mark SGZ/Hilus vs. GCL/MCL boundaries. Full length blots in Supplementary Fig. 9.
Similar articles
- Wnt/β-catenin signalling is dispensable for adult neural stem cell homeostasis and activation.
Austin SHL, Gabarró-Solanas R, Rigo P, Paun O, Harris L, Guillemot F, Urbán N. Austin SHL, et al. Development. 2021 Oct 15;148(20):dev199629. doi: 10.1242/dev.199629. Epub 2021 Oct 19. Development. 2021. PMID: 34557919 Free PMC article. - The role of β-catenin signaling pathway on proliferation of rats neural stem cells after hyperbaric oxygen therapy in vitro.
Zhang XY, Yang YJ, Xu PR, Zheng XR, Wang QH, Chen CF, Yao Y. Zhang XY, et al. Cell Mol Neurobiol. 2011 Jan;31(1):101-9. doi: 10.1007/s10571-010-9559-z. Epub 2010 Oct 1. Cell Mol Neurobiol. 2011. PMID: 20886368 - The Wnt adaptor protein ATP6AP2 regulates multiple stages of adult hippocampal neurogenesis.
Schafer ST, Han J, Pena M, von Bohlen Und Halbach O, Peters J, Gage FH. Schafer ST, et al. J Neurosci. 2015 Mar 25;35(12):4983-98. doi: 10.1523/JNEUROSCI.4130-14.2015. J Neurosci. 2015. PMID: 25810528 Free PMC article. - Age-dependent decline in neurogenesis of the hippocampus and extracellular nucleotides.
Takei Y. Takei Y. Hum Cell. 2019 Apr;32(2):88-94. doi: 10.1007/s13577-019-00241-9. Epub 2019 Feb 7. Hum Cell. 2019. PMID: 30730038 Review. - Regulation of Adult Mammalian Neural Stem Cells and Neurogenesis by Cell Extrinsic and Intrinsic Factors.
Matsubara S, Matsuda T, Nakashima K. Matsubara S, et al. Cells. 2021 May 10;10(5):1145. doi: 10.3390/cells10051145. Cells. 2021. PMID: 34068607 Free PMC article. Review.
Cited by
- Differential adhesion during development establishes individual neural stem cell niches and shapes adult behaviour in Drosophila.
Banach-Latapy A, Rincheval V, Briand D, Guénal I, Spéder P. Banach-Latapy A, et al. PLoS Biol. 2023 Nov 9;21(11):e3002352. doi: 10.1371/journal.pbio.3002352. eCollection 2023 Nov. PLoS Biol. 2023. PMID: 37943883 Free PMC article. - From Youthful Vigor to Aging Decline: Unravelling the Intrinsic and Extrinsic Determinants of Hippocampal Neural Stem Cell Aging.
Jiménez Peinado P, Urbach A. Jiménez Peinado P, et al. Cells. 2023 Aug 17;12(16):2086. doi: 10.3390/cells12162086. Cells. 2023. PMID: 37626896 Free PMC article. Review. - The Adult Neurogenesis Theory of Alzheimer's Disease.
Abbate C. Abbate C. J Alzheimers Dis. 2023;93(4):1237-1276. doi: 10.3233/JAD-221279. J Alzheimers Dis. 2023. PMID: 37182879 Free PMC article. - The Masticatory Activity Interference in Quantitative Estimation of CA1, CA3 and Dentate Gyrus Hippocampal Astrocytes of Aged Murine Models and under Environmental Stimulation.
Cunha Feio Leal MD, Amaral Junior FLD, Silva Arruda BFD, Kurosawa JAA, Vieira AA, Maia JCC, Scalfoni VVB, Silveira Junior AMD, Feijó MO, Albuquerque FBA, Marta MHM, Normando MPN, Silva AGOCD, Trindade FCPD, Siqueira Mendes FCC, Sosthenes MCK. Cunha Feio Leal MD, et al. Int J Mol Sci. 2023 Mar 31;24(7):6529. doi: 10.3390/ijms24076529. Int J Mol Sci. 2023. PMID: 37047502 Free PMC article. - Exploration of the Core Pathways and Potential Targets of Luteolin Treatment on Late-Onset Depression Based on Cerebrospinal Fluid Proteomics.
Liu K, Li H, Zeng N, Li B, Yao G, Wu X, Xu H, Yan C, Wu L. Liu K, et al. Int J Mol Sci. 2023 Feb 9;24(4):3485. doi: 10.3390/ijms24043485. Int J Mol Sci. 2023. PMID: 36834894 Free PMC article.
References
- Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. - PubMed
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. - PubMed
- Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisén J. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous