Natural product disaccharide engineering through tandem glycosyltransferase catalysis reversibility and neoglycosylation - PubMed (original) (raw)
. 2012 Oct 5;14(19):5086-9.
doi: 10.1021/ol3023374. Epub 2012 Sep 17.
Affiliations
- PMID: 22984807
- PMCID: PMC3489467
- DOI: 10.1021/ol3023374
Natural product disaccharide engineering through tandem glycosyltransferase catalysis reversibility and neoglycosylation
Pauline Peltier-Pain et al. Org Lett. 2012.
Abstract
A two-step strategy for disaccharide modulation using vancomycin as a model is reported. The strategy relies upon a glycosyltransferase-catalyzed 'reverse' reaction to enable the facile attachment of an alkoxyamine-bearing sugar to the vancomycin core. Neoglycosylation of the corresponding aglycon led to a novel set of vancomycin 1,6-disaccharide variants. While the in vitro antibacterial properties of corresponding vancomycin 1,6-disaccharide analogs were equipotent to the parent antibiotic, the chemoenzymatic method presented is expected to be broadly applicable.
Figures
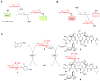
Figure 1
(A) Classical GT reaction in which glycoside formation is thermodynamically favored. (B) GT reaction where an appropriately ‘activated’ glycoside donor (Donor*) shifts the reaction thermodynamics to favor sugar nucleotide formation. (C) One-pot coupled dual-GT-catalyzed reaction which combines GT-driven (OleD) sugar nucleotide synthesis with a subsequent GT-catalyzed (GtfE) glycosylation reaction to ultimately accomplish transglycosylation from a synthetic aromatic glycoside to a targeted aglycon (in this case, the vancomycin aglycon 3).
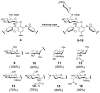
Figure 2
Structures of the natural glycopeptide antibiotics vancomycin (5) and teicoplanin (6) and known natural products containing D-forosamine.
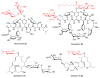
Figure 3
The neoglycosylation of the 6′-alkoxyaminosugar-substituted vancomycin neoaglycon (4) (upper). The products of the neoglycosylation reaction with corresponding conversions based upon HPLC are illustrated (lower). Full characterization of 9–16 is presented in supporting information.
Similar articles
- Exploiting the reversibility of natural product glycosyltransferase-catalyzed reactions.
Zhang C, Griffith BR, Fu Q, Albermann C, Fu X, Lee IK, Li L, Thorson JS. Zhang C, et al. Science. 2006 Sep 1;313(5791):1291-4. doi: 10.1126/science.1130028. Science. 2006. PMID: 16946071 - Deconstructing vancomycin.
Walsh C. Walsh C. Science. 1999 Apr 16;284(5413):442-3. doi: 10.1126/science.284.5413.442. Science. 1999. PMID: 10232990 No abstract available. - Leloir glycosyltransferases of natural product C-glycosylation: structure, mechanism and specificity.
Tegl G, Nidetzky B. Tegl G, et al. Biochem Soc Trans. 2020 Aug 28;48(4):1583-1598. doi: 10.1042/BST20191140. Biochem Soc Trans. 2020. PMID: 32657344 Review. - Disaccharide analogs as probes for glycosyltransferases in Mycobacterium tuberculosis.
Pathak AK, Pathak V, Seitz L, Gurcha SS, Besra GS, Riordan JM, Reynolds RC. Pathak AK, et al. Bioorg Med Chem. 2007 Aug 15;15(16):5629-50. doi: 10.1016/j.bmc.2007.04.012. Epub 2007 Apr 10. Bioorg Med Chem. 2007. PMID: 17544276 Free PMC article. - The structural biology of enzymes involved in natural product glycosylation.
Singh S, Phillips GN Jr, Thorson JS. Singh S, et al. Nat Prod Rep. 2012 Oct;29(10):1201-37. doi: 10.1039/c2np20039b. Epub 2012 Jun 12. Nat Prod Rep. 2012. PMID: 22688446 Free PMC article. Review.
Cited by
- Naturally Occurring Organohalogen Compounds-A Comprehensive Review.
Gribble GW. Gribble GW. Prog Chem Org Nat Prod. 2023;121:1-546. doi: 10.1007/978-3-031-26629-4_1. Prog Chem Org Nat Prod. 2023. PMID: 37488466 Review. - Promiscuous Enzymes for Residue-Specific Peptide and Protein Late-Stage Functionalization.
Alexander AK, Elshahawi SI. Alexander AK, et al. Chembiochem. 2023 Sep 1;24(17):e202300372. doi: 10.1002/cbic.202300372. Epub 2023 Jul 19. Chembiochem. 2023. PMID: 37338668 Free PMC article. Review. - Emulating nonribosomal peptides with ribosomal biosynthetic strategies.
Mordhorst S, Ruijne F, Vagstad AL, Kuipers OP, Piel J. Mordhorst S, et al. RSC Chem Biol. 2022 Dec 6;4(1):7-36. doi: 10.1039/d2cb00169a. eCollection 2023 Jan 4. RSC Chem Biol. 2022. PMID: 36685251 Free PMC article. Review. - Controllable Iterative β-Glucosylation from UDP-Glucose by Bacillus cereus Glycosyltransferase GT1: Application for the Synthesis of Disaccharide-Modified Xenobiotics.
Jung J, Schachtschabel D, Speitling M, Nidetzky B. Jung J, et al. J Agric Food Chem. 2021 Dec 8;69(48):14630-14642. doi: 10.1021/acs.jafc.1c05788. Epub 2021 Nov 24. J Agric Food Chem. 2021. PMID: 34817995 Free PMC article. - Enzyme-mediated bioorthogonal technologies: catalysts, chemoselective reactions and recent methyltransferase applications.
Jalali E, Thorson JS. Jalali E, et al. Curr Opin Biotechnol. 2021 Jun;69:290-298. doi: 10.1016/j.copbio.2021.02.010. Epub 2021 Apr 24. Curr Opin Biotechnol. 2021. PMID: 33901763 Free PMC article. Review.
References
- Gantt RW, Peltier-Pain P, Thorson JS. Nat Prod Reports. 2011;28:1811. - PubMed
- Chang A, Singh S, Phillips GN, Jr, Thorson JS. Curr Opin Biotechnol. 2011;22:800. - PMC - PubMed
- Palcic M. Curr Opin Chem Biol. 2011;15:226. - PubMed
- Lairson L, Henrissat B, Davies GJ, Withers SG. Annu Rev Biochem. 2008;77:521. - PubMed
- Erb A, Weiss H, Harle J, Bechthold A. Phytochemistry. 2009;70:1812. - PubMed
- Zhang C, Griffith BR, Fu Q, Albermann C, Fu X, Lee I-K, Li L, Thorson JS. Science. 2006;313:1291. - PubMed
- Modolo LV, Escamilla-Treviño LL, Dixon RA, Wang X. FEBS Lett. 2009;583:2131. - PubMed
- Zhang C, Moretti R, Jiang J, Thorson JS. ChemBioChem. 2008;9:2506. - PMC - PubMed
- Minami A, Kakinuma K, Eguchi T. Tetrahedron Lett. 2005;46:6187.
- Zhang C, Albermann C, Fu X, Thorson JSJ. Am Chem Soc. 2006;128:16420. - PubMed
- Lairson LL, Wakarchuk WW, Withers SG. Chem Commun. 2007:365. - PubMed
- Lougheed B, Ly HD, Wakarchuk WW, Withers SG. J Biol Chem. 1999;274:37717. - PubMed
- Peri F, Dumy P, Mutter M. Tetrahedron. 1998;54:12269.
- Peri F, Deutman A, La Ferla B, Nicotra F. Chem Commun. 2002;1504 - PubMed
- Peri F, Jimenez-Barbero J, Garcia-Aparicio V, Tvaroska I, Nicotra F. Chem-Eur J. 2004;10:1433. - PubMed
- Griffith BR, Langenhan JM, Thorson JS. Curr Opin Biotechnol. 2005;16:622. - PubMed
- Langenhan JM, Peters NR, Guzei IA, Hoffman FM, Thorson JS. Proc Natl Acad Sci U S A. 2005;102:12305. - PMC - PubMed
- Griffith BR, Krepel C, Fu X, Blanchard S, Ahmed A, Edmiston CE, Thorson JS. J Am Chem Soc. 2007;129:8150. - PubMed
- Ahmed A, Peters NR, Fitzgerald MK, Watson JA, Jr, Hoffman FM, Thorson JS. J Am Chem Soc. 2006;128:14224. - PubMed
- Goff RD, Thorson JS. J Med Chem. 2010;53:8129. - PMC - PubMed
- Goff RD, Thorson JS. Chem Med Chem. 2011;6:7774. - PubMed
- Langenhan JM, Engle JM, Slevin LK, Fay LR, Lucker RW, Smith KR, Endo MM. Bioorg Med Chem Lett. 2008;18:670. - PubMed
- Peltier-Pain P, Timmons SC, Grandemange A, Benoit E, Thorson JS. Chem Med Chem. 2011;6:1347. - PMC - PubMed
- Goff RD, Thorson JS. Org Lett. 2009;11:461. - PMC - PubMed
- Goff RD, Thorson JS. Org Lett. 2012;14:2454. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000117/TR/NCATS NIH HHS/United States
- R37 AI052218/AI/NIAID NIH HHS/United States
- R01 AI052218/AI/NIAID NIH HHS/United States
- AI52218/AI/NIAID NIH HHS/United States
- UL1 TR001998/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical